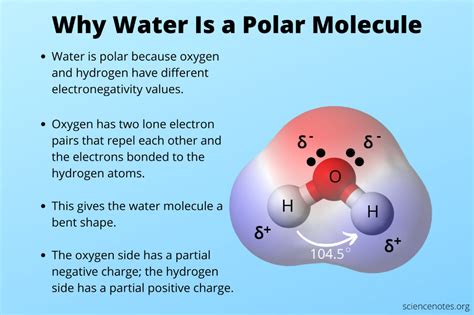
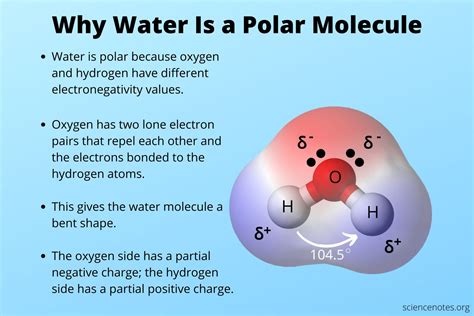
Water, a compound essential for life, exhibits unique properties that set it apart from other substances. One of its most distinctive characteristics is its polarity. The term "polar" refers to the separation of electric charge within a molecule, resulting in a molecule with an electric dipole moment. Water's polarity is due to the difference in electronegativity between its oxygen and hydrogen atoms. Oxygen, being more electronegative, pulls electrons closer to itself, creating a partial negative charge. Conversely, the hydrogen atoms have a partial positive charge, as they lose electrons to the oxygen atom. This separation of charges within the molecule is the essence of water's polarity.
Understanding Polarity in Water Molecules
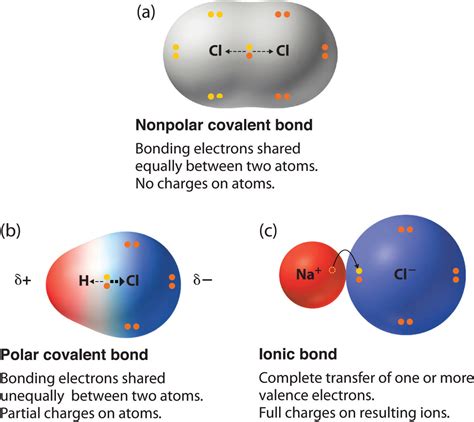
The molecular structure of water, composed of one oxygen atom bonded to two hydrogen atoms, is bent or V-shaped. This geometry contributes to the molecule’s overall polarity. The oxygen atom, with its higher electronegativity, draws the shared electrons closer, creating a slight imbalance in the distribution of electrons. As a result, the oxygen end of the molecule carries a partial negative charge (δ-), while the hydrogen ends carry partial positive charges (δ+). This dipole moment, a measure of the separation of charge, is a fundamental aspect of water’s chemical properties and plays a crucial role in its interactions with other molecules.
Electronegativity and Its Role in Polarity
Electronegativity, a measure of an atom’s ability to attract electrons in a covalent bond, is pivotal in understanding the polarity of water. Oxygen’s electronegativity value is approximately 3.44 on the Pauling scale, significantly higher than hydrogen’s value of about 2.20. This difference leads to the unequal sharing of electrons between oxygen and hydrogen, resulting in the partial charges that define water’s polarity. The electronegativity difference is not only crucial for water’s polarity but also influences its reactivity and ability to form hydrogen bonds, which are essential for many biological processes.
| Atom | Electronegativity Value |
|---|---|
| Oxygen (O) | 3.44 |
| Hydrogen (H) | 2.20 |
Implications of Water’s Polarity

Water’s polarity has far-reaching implications that affect its interactions at the molecular, biological, and environmental levels. One of the most significant effects of polarity is the formation of hydrogen bonds. Hydrogen bonds are weak electrostatic attractions between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another. These bonds are crucial for the structure of water in its solid (ice) and liquid states, influencing properties such as viscosity, surface tension, and the melting and boiling points. Furthermore, hydrogen bonding plays a critical role in the structure and function of biomolecules, including proteins and DNA, where it helps stabilize their three-dimensional conformations.
Biological and Environmental Significance
The biological and environmental significance of water’s polarity cannot be overstated. In living organisms, water’s polarity allows it to dissolve and transport a wide range of substances, from nutrients and minerals to waste products. This solvent property is vital for metabolic processes, including digestion, absorption, and the transport of nutrients and oxygen to cells. Environmentally, water’s polarity influences its role in weather patterns, the water cycle, and the formation of clouds and precipitation. The ability of water to absorb and release heat energy, due in part to its polarity and hydrogen bonding, helps regulate Earth’s climate and weather patterns.
Key Points
- Water's polarity arises from the difference in electronegativity between oxygen and hydrogen atoms, leading to a partial negative charge on oxygen and partial positive charges on hydrogen.
- The polarity of water molecules enables the formation of hydrogen bonds, which are essential for the structure and properties of water and its interactions with other molecules.
- Water's solvent properties, influenced by its polarity, allow it to dissolve a wide variety of substances, making it crucial for biological processes and environmental cycles.
- The implications of water's polarity extend to its physical and chemical properties, including its high surface tension, boiling point, and specific heat capacity.
- Understanding water's polarity is fundamental for appreciating its role in biological systems, environmental processes, and its unique position in the universe as a solvent and medium for life.
What is the primary reason for water's polarity?
+The primary reason for water's polarity is the difference in electronegativity between the oxygen and hydrogen atoms in a water molecule. Oxygen, being more electronegative, pulls electrons closer to itself, creating a partial negative charge, while the hydrogen atoms have a partial positive charge.
How does the polarity of water influence its ability to form hydrogen bonds?
+The polarity of water molecules allows them to form hydrogen bonds with each other. The partial positive charge on the hydrogen atoms of one water molecule is attracted to the partial negative charge on the oxygen atom of another water molecule, forming a weak electrostatic bond known as a hydrogen bond.
What are some biological implications of water's polarity?
+Water's polarity has several biological implications. It allows water to dissolve and transport a wide range of substances, which is vital for metabolic processes. Additionally, the polarity of water influences the structure and function of biomolecules, such as proteins and DNA, by facilitating the formation of hydrogen bonds that stabilize their three-dimensional conformations.
In conclusion, water’s polarity is a fundamental property that underlies many of its unique characteristics and plays a crucial role in its interactions with other molecules. Understanding the origins and implications of water’s polarity is essential for appreciating its significance in biological, environmental, and chemical contexts. As a solvent, reactant, and medium for life, water’s polarity makes it an indispensable component of our planet’s ecosystem, influencing processes from the molecular to the global scale.