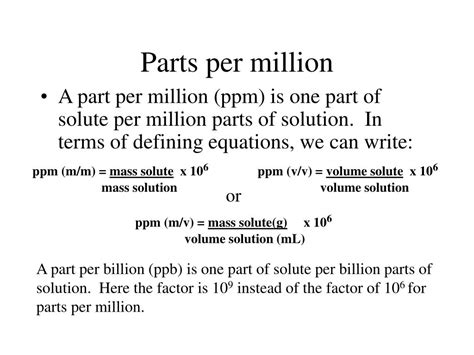
The Parts Per Million (PPM) formula is a widely used mathematical expression in various fields, including chemistry, environmental science, and engineering. It represents the ratio of a component's quantity to the total mixture's quantity, expressed in units of one million. Understanding the PPM formula is essential for accurately calculating concentrations, solubilities, and contamination levels in different substances. In this article, we will delve into the PPM formula, its derivation, applications, and examples, providing a comprehensive overview of its significance and usage.
Derivation of the PPM Formula
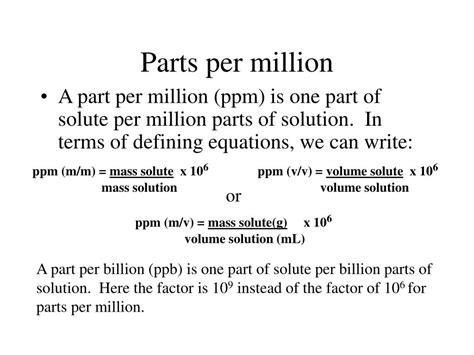
The PPM formula is derived from the concept of proportion, where a small quantity of a substance is compared to the total quantity of the mixture. Mathematically, it can be represented as:
PPM = (Component quantity / Total mixture quantity) x 1,000,000
This formula can be applied to various units, such as grams, milligrams, liters, or cubic meters, as long as the units are consistent. For instance, if you have 2 grams of a substance in a 100-liter solution, the PPM calculation would be:
PPM = (2 grams / 100 liters) x 1,000,000 = 20 PPM
This means that the substance has a concentration of 20 parts per million in the solution.
Applications of the PPM Formula
The PPM formula has numerous applications in different fields, including:
- Environmental monitoring: PPM is used to measure the concentration of pollutants in air, water, and soil, helping to assess the level of contamination and develop strategies for mitigation.
- Chemical analysis: PPM is employed to determine the concentration of chemicals in solutions, allowing for the calculation of solubilities, reaction rates, and equilibrium constants.
- Industrial processes: PPM is used to monitor and control the concentration of substances in industrial processes, such as water treatment, pharmaceutical manufacturing, and food processing.
| Application | PPM Range |
|---|---|
| Drinking water quality | 0.01-10 PPM |
| Air pollution monitoring | 0.1-100 PPM |
| Chemical synthesis | 1-1000 PPM |
Key Considerations and Limitations

While the PPM formula is a valuable tool for calculating concentrations, there are some key considerations and limitations to keep in mind:
Unit consistency: As mentioned earlier, it’s crucial to ensure that the units used for the component quantity and total mixture quantity are consistent to avoid errors in the calculation.
Measurement accuracy: The accuracy of the PPM calculation depends on the accuracy of the measurements used. Small errors in measurement can result in significant errors in the calculated PPM value.
Contextual interpretation: PPM values should be interpreted in the context of the specific application or field. For example, a PPM value of 10 may be considered high in one context but low in another.
Key Points
- The PPM formula is used to calculate the concentration of a component in a mixture, expressed in units of one million.
- The formula is derived from the concept of proportion and can be applied to various units.
- PPM has numerous applications in environmental monitoring, chemical analysis, and industrial processes.
- Unit consistency, measurement accuracy, and contextual interpretation are essential considerations when working with PPM calculations.
- PPM values should be interpreted in the context of the specific application or field.
In conclusion, the PPM formula is a powerful tool for calculating concentrations and solubilities in various fields. By understanding the derivation, applications, and limitations of the PPM formula, professionals can make informed decisions and develop effective strategies for monitoring and controlling concentrations in different substances.
What is the difference between PPM and percentage?
+PPM and percentage are both units of concentration, but they differ in their scale. Percentage is a unit of concentration that represents the ratio of a component’s quantity to the total mixture quantity, expressed as a fraction of 100. PPM, on the other hand, represents the ratio of a component’s quantity to the total mixture quantity, expressed in units of one million.
How do I convert PPM to percentage?
+To convert PPM to percentage, divide the PPM value by 10,000. For example, 100 PPM is equivalent to 0.01%.
What are some common applications of PPM in environmental monitoring?
+PPM is commonly used in environmental monitoring to measure the concentration of pollutants in air, water, and soil. Some examples include monitoring the concentration of particulate matter in air, measuring the levels of heavy metals in water, and assessing the concentration of pesticides in soil.