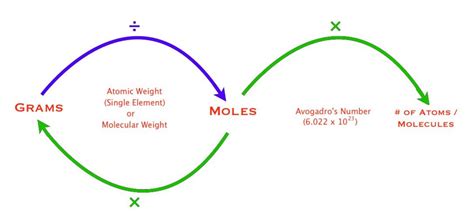
The concept of a mole, a fundamental unit of measurement in chemistry, is often met with confusion, especially when it comes to understanding the relationship between moles and grams. The phrase "6 grams in a mole" is not entirely accurate, as it oversimplifies the complex relationship between these two units. To grasp this concept, it's essential to delve into the definitions and applications of both moles and grams.
Understanding Moles and Grams
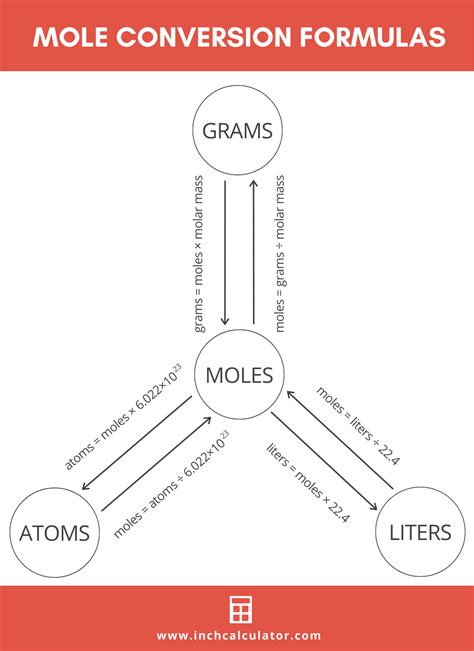
A mole (mol) is the base unit of amount of substance in the International System of Units (SI) and is defined as the amount of substance that contains as many particles (atoms, molecules, ions, etc.) as there are atoms in 0.012 kilograms of carbon-12. This number, known as the Avogadro’s constant (NA), is approximately 6.022 x 10^23 particles. On the other hand, a gram (g) is a unit of mass, where 1 gram is one-thousandth of a kilogram.
The Mole-Gram Relationship
The relationship between moles and grams is not straightforward because it depends on the substance in question. The molar mass of a substance, which is the mass of one mole of that substance, is expressed in grams per mole (g/mol). For example, the molar mass of carbon-12 is exactly 12 grams per mole, meaning that one mole of carbon-12 weighs 12 grams. However, for other elements or compounds, the molar mass varies. For instance, the molar mass of oxygen (O2) is approximately 32 grams per mole, and the molar mass of water (H2O) is about 18 grams per mole.
| Substance | Molar Mass (g/mol) |
|---|---|
| Carbon-12 | 12.0000 |
| Oxygen (O2) | 31.9988 |
| Water (H2O) | 18.0153 |
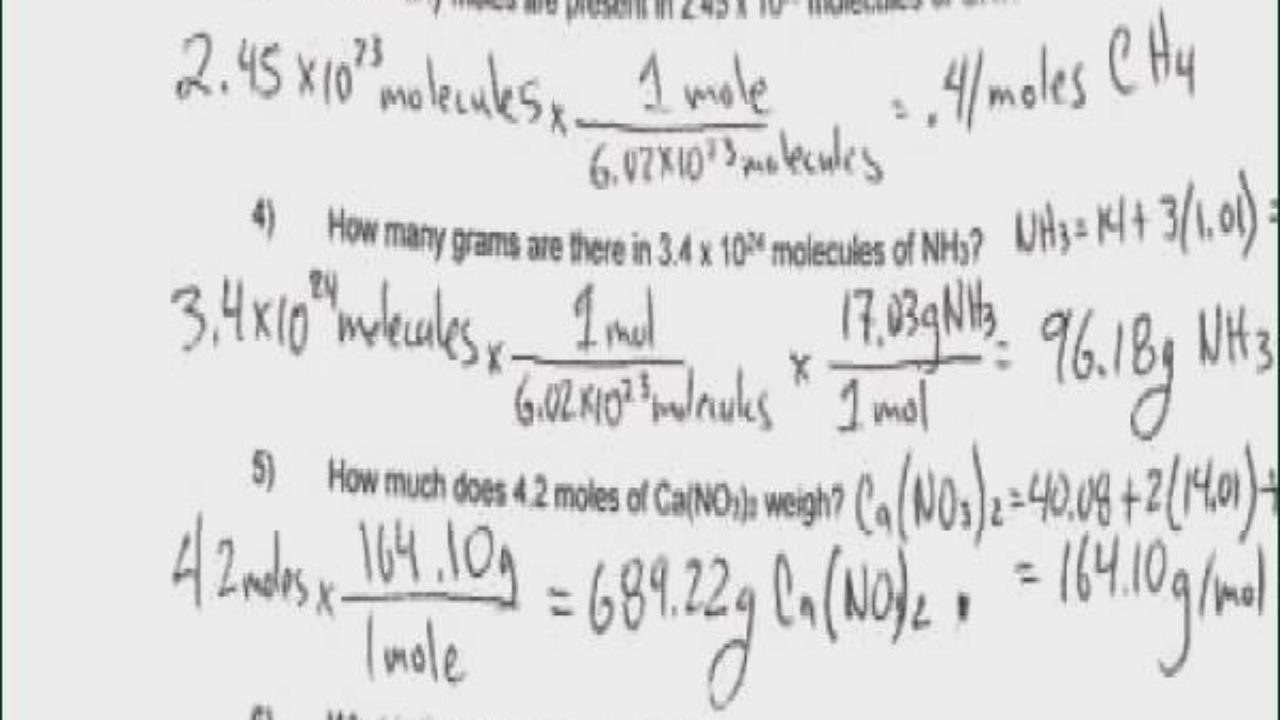
Applications and Implications
The mole-gram relationship has significant implications in various chemical applications, including the preparation of solutions, the calculation of yields in chemical reactions, and the determination of the composition of mixtures. For example, knowing the molar mass of a substance allows chemists to calculate the number of moles of that substance in a given mass, which is essential for preparing solutions of known concentration.
Calculating Moles from Grams
The calculation of moles from grams involves dividing the given mass in grams by the molar mass of the substance. This calculation is straightforward but requires accurate knowledge of the molar mass. For instance, to find the number of moles of sodium chloride (NaCl) in 100 grams, given that the molar mass of NaCl is approximately 58.44 g/mol, one would divide 100 grams by 58.44 g/mol.
Key Points
- The mole is a unit of amount of substance, defined as the amount containing as many particles as there are atoms in 0.012 kg of carbon-12.
- The gram is a unit of mass, with 1 gram being one-thousandth of a kilogram.
- The relationship between moles and grams depends on the molar mass of the substance, which varies.
- Understanding this relationship is crucial for calculations in chemistry, especially in stoichiometry.
- Calculations involving moles and grams are essential for preparing solutions, determining reaction yields, and analyzing mixtures.
In conclusion, while the phrase "6 grams in a mole" might seem simple, it belies the complexity of the relationship between moles and grams. This relationship, governed by the molar mass of a substance, is fundamental to chemistry and has wide-ranging applications. By grasping this concept, individuals can better understand and apply chemical principles in various contexts.
What is the definition of a mole in chemistry?
+A mole is the amount of substance that contains as many particles (atoms, molecules, ions, etc.) as there are atoms in 0.012 kilograms of carbon-12, which is approximately 6.022 x 10^23 particles.
How do you calculate the number of moles from a given mass in grams?
+To calculate the number of moles from a given mass in grams, you divide the mass in grams by the molar mass of the substance.
Why is understanding the mole-gram relationship important in chemistry?
+Understanding the mole-gram relationship is crucial for accurate calculations in chemistry, particularly in stoichiometry, solution preparation, and the analysis of chemical reactions and mixtures.