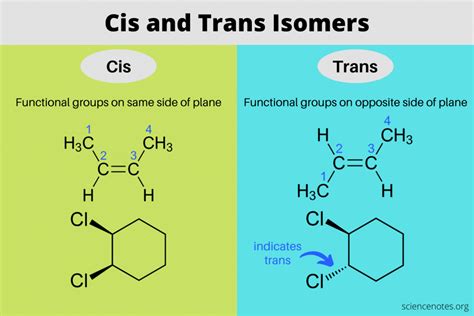
The concept of CIS TRANS isomers is a fundamental aspect of organic chemistry, particularly in the realm of stereochemistry. CIS TRANS isomerism occurs in molecules that have a double bond or a ring structure, leading to the possibility of different spatial arrangements of atoms or groups of atoms. Understanding how CIS TRANS isomers work is crucial for predicting the physical and chemical properties of molecules, as well as their potential applications in various fields. In this article, we will delve into the world of CIS TRANS isomers, exploring five key ways in which they function and the implications of their unique properties.
Key Points
- CIS TRANS isomerism arises from the restricted rotation around a double bond or a ring structure.
- The CIS and TRANS configurations influence the physical properties of molecules, such as boiling points and melting points.
- CIS TRANS isomers can exhibit different chemical reactivities due to the distinct spatial arrangements of their atoms or groups.
- The biological activities of molecules can be significantly affected by their CIS TRANS configurations.
- The synthesis of CIS TRANS isomers requires careful control of reaction conditions to achieve the desired configuration.
Introduction to CIS TRANS Isomerism
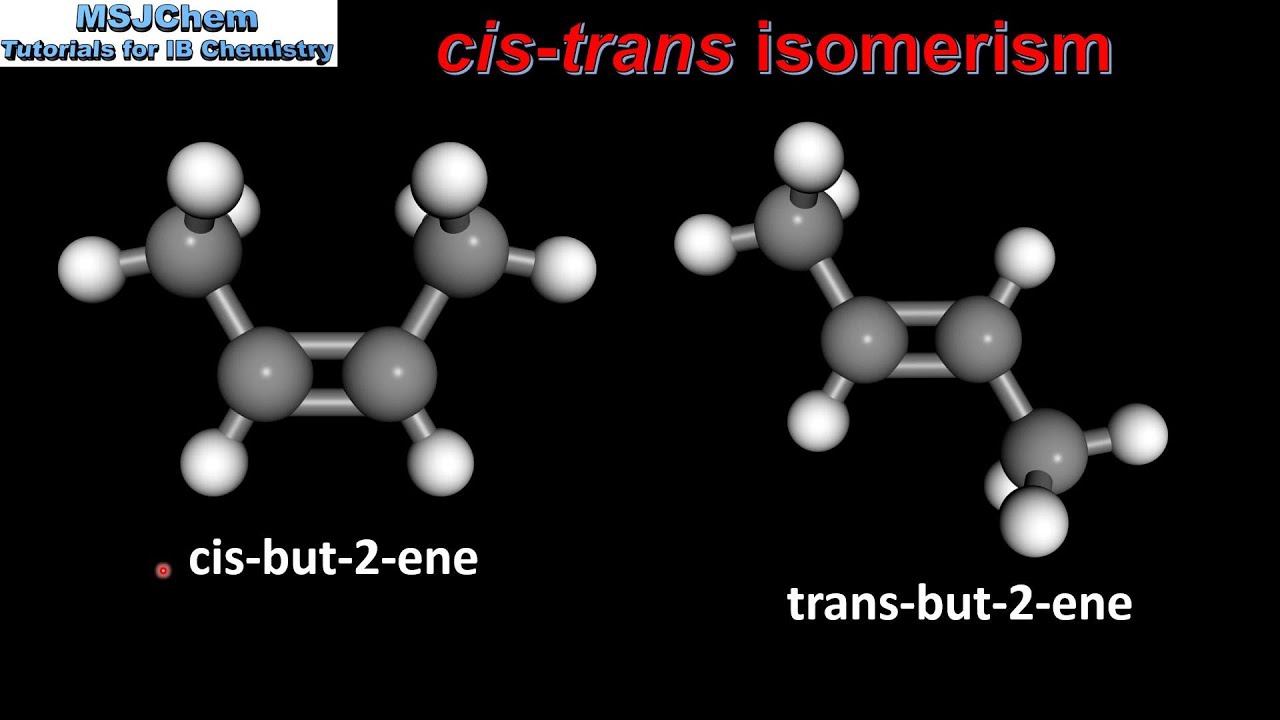
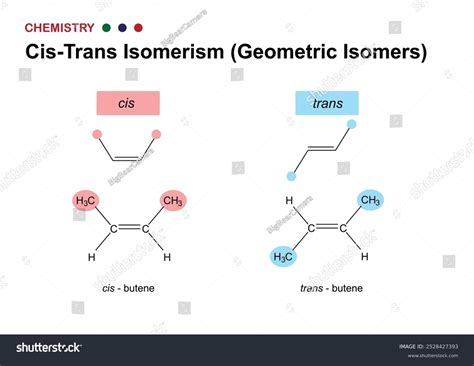
CIS TRANS isomerism is a type of stereoisomerism that occurs in molecules with a double bond between two atoms, typically carbon atoms, or in molecules with a ring structure. The double bond restricts the rotation of the atoms or groups attached to the double bond, leading to fixed positions in space. This restriction gives rise to two possible configurations: CIS, where similar atoms or groups are on the same side of the double bond, and TRANS, where they are on opposite sides. The CIS and TRANS configurations can significantly influence the physical and chemical properties of molecules, making understanding and controlling CIS TRANS isomerism crucial in chemistry.
CIS TRANS Isomerism in Alkenes
Alkenes, which are hydrocarbons containing at least one carbon-to-carbon double bond, are a primary class of compounds exhibiting CIS TRANS isomerism. The presence of the double bond restricts the rotation of the carbon atoms, resulting in CIS and TRANS isomers. For example, 1,2-dichloroethene (C2H2Cl2) can exist as CIS-1,2-dichloroethene and TRANS-1,2-dichloroethene. The CIS isomer has both chlorine atoms on the same side of the double bond, while the TRANS isomer has them on opposite sides. This difference in configuration affects the molecule’s polarity, boiling point, and reactivity.
| Isomer | Boiling Point (°C) |
|---|---|
| CIS-1,2-dichloroethene | 60.3 |
| TRANS-1,2-dichloroethene | 47.5 |
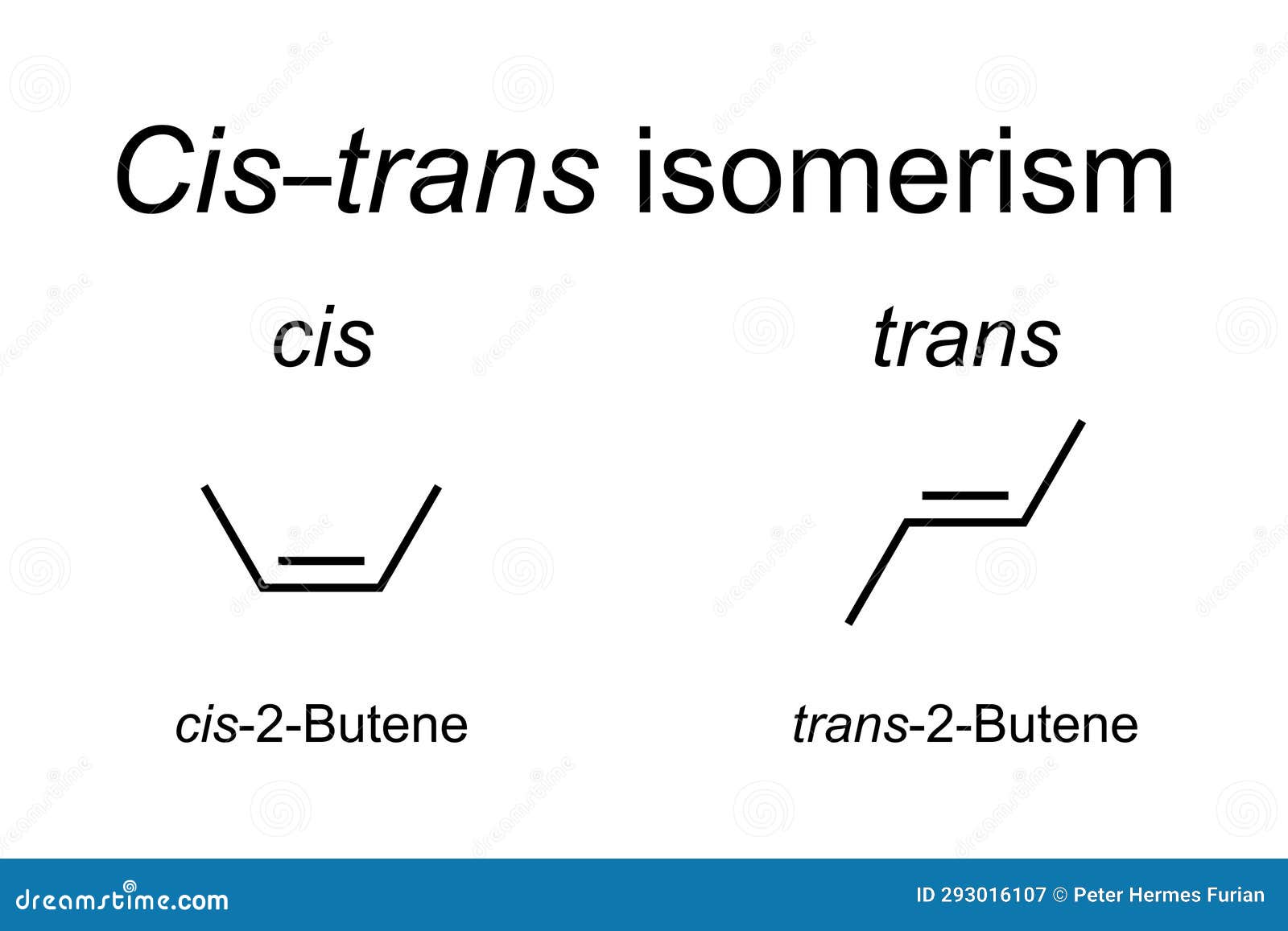
Chemical Reactivity of CIS TRANS Isomers
The chemical reactivity of CIS TRANS isomers can differ significantly due to the distinct spatial arrangements of their atoms or groups. The accessibility of reactive sites and the orientation of functional groups can influence how a molecule interacts with other reactants. For instance, in the hydrogenation of alkenes, the CIS or TRANS configuration can affect the approach of the hydrogen molecule to the double bond, potentially leading to different products or reaction rates. Understanding these differences is essential for predicting and controlling the outcomes of chemical reactions.
Biological Activity and CIS TRANS Isomerism
The biological activities of molecules, including their interactions with enzymes, receptors, and other biomolecules, can be profoundly influenced by their CIS TRANS configurations. The shape and polarity of a molecule, which are determined by its CIS or TRANS configuration, can affect its ability to bind to a specific site on an enzyme or receptor. This is particularly relevant in pharmacology, where the efficacy and specificity of drugs can depend on their stereochemistry. For example, the CIS isomer of a certain drug might be more potent or have fewer side effects than its TRANS counterpart.
Synthesis of CIS TRANS Isomers
The synthesis of CIS TRANS isomers requires careful control of reaction conditions to achieve the desired configuration. Various methods, including catalytic hydrogenation, electrophilic addition reactions, and photochemical reactions, can be employed to synthesize alkenes with specific CIS or TRANS configurations. The choice of reaction conditions, such as temperature, pressure, and the presence of catalysts, can influence the outcome of the reaction and the ratio of CIS to TRANS products. Understanding the mechanisms of these reactions and how to control them is essential for the synthesis of compounds with specific stereochemical properties.
What is the primary factor influencing CIS TRANS isomerism in molecules?
+The primary factor is the restricted rotation around a double bond or a ring structure, which leads to fixed positions of atoms or groups in space.
How do CIS TRANS isomers differ in terms of physical properties?
+CIS and TRANS isomers can exhibit different boiling points, melting points, and polarities due to their distinct configurations.
What is the significance of CIS TRANS isomerism in biological systems?
+The CIS TRANS configuration can significantly affect the biological activity of molecules, including their interactions with enzymes and receptors, which is crucial in pharmacology and drug development.
In conclusion, CIS TRANS isomerism is a fundamental concept in organic chemistry that influences the physical and chemical properties of molecules. The distinct spatial arrangements of atoms or groups in CIS and TRANS configurations can affect their reactivity, biological activity, and synthesis. Understanding and controlling CIS TRANS isomerism is essential for predicting and manipulating the properties of molecules, which has significant implications for various fields, including chemistry, pharmacology, and materials science. By recognizing the importance of stereochemistry and the specific ways in which CIS TRANS isomers work, researchers and scientists can design and develop new compounds with tailored properties, contributing to advancements in science and technology.