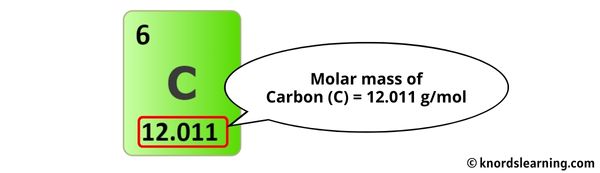
The concept of molar mass is fundamental in chemistry, as it allows us to understand the quantitative aspects of chemical reactions and compositions. One such compound that is often of interest is propane, with the chemical formula C3H8. To calculate the molar mass of propane, we need to consider the atomic masses of carbon (C) and hydrogen (H). The atomic mass of carbon is approximately 12.01 g/mol, and the atomic mass of hydrogen is approximately 1.008 g/mol.
Understanding the Formula for Molar Mass Calculation
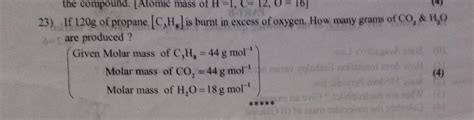
The formula to calculate the molar mass of a compound is the sum of the atomic masses of its constituent atoms. For propane (C3H8), this means we add the atomic masses of three carbon atoms and eight hydrogen atoms. The calculation is as follows: (3 * 12.01) for carbon + (8 * 1.008) for hydrogen.
Step-by-Step Calculation of Propane’s Molar Mass
To find the molar mass of propane, we follow these steps:
- Calculate the total mass contributed by carbon: 3 * 12.01 g/mol = 36.03 g/mol
- Calculate the total mass contributed by hydrogen: 8 * 1.008 g/mol = 8.064 g/mol
- Add these two values together to get the molar mass of propane: 36.03 g/mol + 8.064 g/mol = 44.094 g/mol
| Element | Number of Atoms | Atomic Mass (g/mol) | Total Mass (g/mol) |
|---|---|---|---|
| Carbon (C) | 3 | 12.01 | 36.03 |
| Hydrogen (H) | 8 | 1.008 | 8.064 |
| Total | - | - | 44.094 |
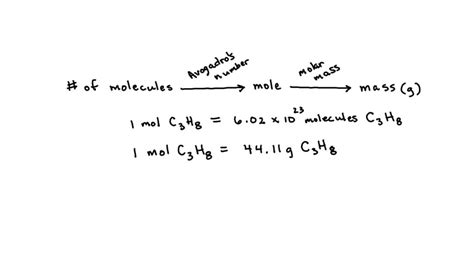
Key Points
- The molar mass of propane (C3H8) is calculated by summing the atomic masses of its constituent atoms: 3 carbon atoms and 8 hydrogen atoms.
- The atomic mass of carbon is approximately 12.01 g/mol, and the atomic mass of hydrogen is approximately 1.008 g/mol.
- The calculation involves multiplying the number of atoms of each element by its atomic mass and then summing these values: (3 * 12.01) + (8 * 1.008) = 44.094 g/mol.
- Understanding the molar mass of compounds like propane is vital for quantitative chemistry, including the calculation of reactant and product amounts in chemical reactions.
- Always refer to the most current and accurate atomic masses to ensure precision in molar mass calculations.
Applications of Propane’s Molar Mass
The molar mass of propane has practical applications in various fields, including chemistry, engineering, and environmental science. For instance, in combustion reactions, knowing the molar mass of propane allows for the calculation of the stoichiometric amounts of oxygen required for complete combustion and the amounts of carbon dioxide and water produced as products.
Environmental Considerations
Propane, as a fossil fuel, contributes to greenhouse gas emissions when burned. The molar mass is useful in calculating the carbon footprint of propane combustion, helping in the assessment of environmental impact and the development of strategies to reduce emissions.
In conclusion, the molar mass of propane, calculated as 44.094 g/mol, is a fundamental piece of information for understanding the chemical and physical properties of this compound. Its applications range from industrial processes to environmental assessments, highlighting the importance of accurate molar mass calculations in chemistry.
What is the primary use of calculating the molar mass of a compound like propane?
+The primary use is to understand the quantitative aspects of chemical reactions and compositions, allowing for the calculation of reactant and product amounts.
How does the molar mass of propane relate to its environmental impact?
+The molar mass of propane is used in calculating the carbon footprint of its combustion, which is essential for assessing environmental impact and developing strategies to reduce emissions.
What are the key elements in calculating the molar mass of propane?
+The key elements are the atomic masses of carbon (approximately 12.01 g/mol) and hydrogen (approximately 1.008 g/mol), and the formula of propane (C3H8), which indicates 3 carbon atoms and 8 hydrogen atoms.