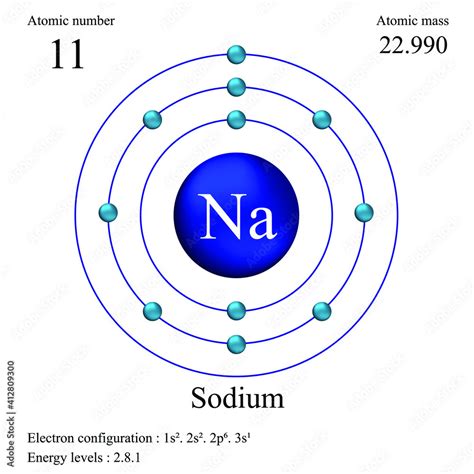
The sodium atomic mass value is a fundamental constant in chemistry, representing the total number of protons and neutrons in the nucleus of a sodium atom. As one of the most abundant elements in the universe, sodium plays a crucial role in various biological, chemical, and industrial processes. The atomic mass of sodium is 22.98976928 u (unified atomic mass units), which is a precise value determined through various experimental methods and theoretical calculations.
The determination of sodium's atomic mass involves the use of mass spectrometry, a technique that measures the mass-to-charge ratio of ions. By analyzing the mass spectra of sodium isotopes, scientists can calculate the average atomic mass of the element. The most common isotopes of sodium are sodium-23 (99.85% abundance) and sodium-22 (0.01% abundance), with smaller amounts of other isotopes present. The weighted average of these isotopic masses yields the standard atomic weight of sodium, which is 22.9898 u.
Key Points
- The atomic mass of sodium is 22.98976928 u, representing the total number of protons and neutrons in the nucleus.
- The most common isotopes of sodium are sodium-23 (99.85% abundance) and sodium-22 (0.01% abundance).
- The standard atomic weight of sodium is 22.9898 u, calculated as the weighted average of its isotopic masses.
- Mass spectrometry is a key technique used to determine the atomic mass of sodium and other elements.
- Understanding the atomic mass of sodium is essential for various applications in chemistry, physics, and biology.
Isotopic Abundance and Atomic Mass Calculation

The calculation of sodium’s atomic mass involves the use of isotopic abundance data, which represents the relative amounts of each isotope present in a sample. By multiplying the mass of each isotope by its abundance and summing these values, scientists can determine the average atomic mass of the element. The following table illustrates the isotopic abundance of sodium and the corresponding atomic masses:
| Isotope | Abundance (%) | Atomic Mass (u) |
|---|---|---|
| Sodium-22 | 0.01 | 21.9944374 |
| Sodium-23 | 99.85 | 22.98976928 |
| Sodium-24 | 0.01 | 23.9909628 |
| Sodium-25 | 0.01 | 24.989801 |
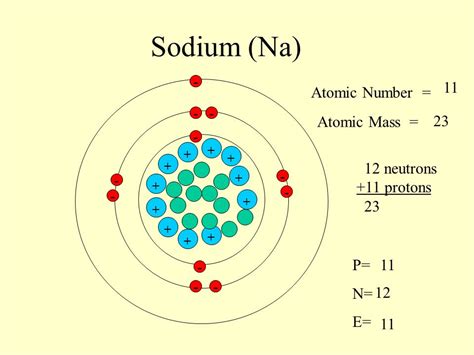
Applications of Sodium’s Atomic Mass
The atomic mass of sodium has numerous applications in various fields, including chemistry, physics, and biology. In chemistry, the atomic mass of sodium is used to calculate the molecular weight of compounds containing sodium, such as sodium chloride (NaCl) and sodium hydroxide (NaOH). In physics, the atomic mass of sodium is used to study the properties of sodium isotopes and their applications in nuclear physics. In biology, the atomic mass of sodium is essential for understanding the role of sodium in biological processes, such as nerve impulse transmission and muscle contraction.
Experimental Methods for Determining Atomic Mass
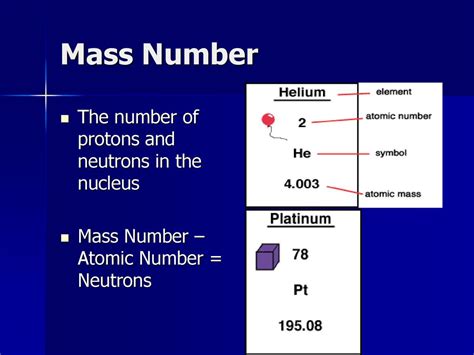
Several experimental methods are used to determine the atomic mass of sodium, including mass spectrometry, nuclear magnetic resonance (NMR) spectroscopy, and X-ray fluorescence (XRF) spectroscopy. Mass spectrometry is the most commonly used method, as it provides high accuracy and precision in measuring the mass-to-charge ratio of ions. NMR spectroscopy and XRF spectroscopy are also used to determine the atomic mass of sodium, although they are less commonly employed due to their lower accuracy and precision compared to mass spectrometry.
The choice of experimental method depends on the specific application and the required level of accuracy. For example, mass spectrometry is often used in high-precision applications, such as the production of isotopically enriched sodium, while NMR spectroscopy and XRF spectroscopy may be used in lower-precision applications, such as the analysis of sodium-containing compounds.
Theoretical Calculations of Atomic Mass
Theoretical calculations of atomic mass are based on the principles of quantum mechanics and the nuclear shell model. These calculations involve the use of complex mathematical formulas and computational methods to predict the atomic mass of sodium and other elements. Theoretical calculations are essential for understanding the underlying physics of atomic mass and for predicting the properties of exotic nuclei and superheavy elements.
Theoretical calculations of atomic mass are also used to validate experimental results and to provide a framework for understanding the systematics of atomic mass. By comparing theoretical predictions with experimental results, scientists can refine their understanding of the underlying physics and improve the accuracy of their calculations.
What is the atomic mass of sodium?
+The atomic mass of sodium is 22.98976928 u, representing the total number of protons and neutrons in the nucleus.
How is the atomic mass of sodium calculated?
+The atomic mass of sodium is calculated as the weighted average of its isotopic masses, using the relative abundance of each isotope.
What are the applications of sodium's atomic mass?
+The atomic mass of sodium has numerous applications in chemistry, physics, and biology, including the calculation of molecular weights, the study of nuclear physics, and the understanding of biological processes.
In conclusion, the atomic mass of sodium is a fundamental constant that plays a critical role in various fields, including chemistry, physics, and biology. The precise determination of sodium’s atomic mass is essential for understanding the properties of sodium and its applications in industry and research. By combining experimental methods and theoretical calculations, scientists can refine their understanding of the atomic mass of sodium and its role in the natural world.