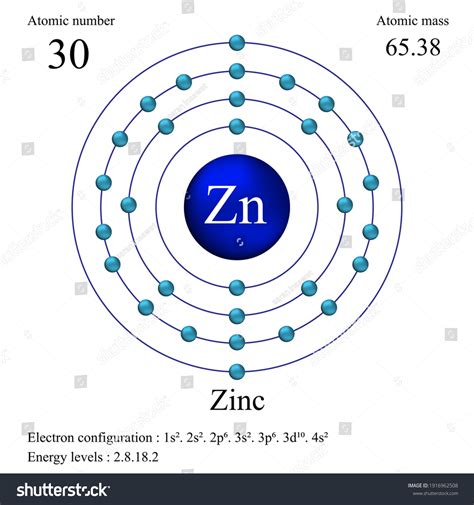
The zinc element, denoted by the symbol Zn, is a transition metal that can be found on the periodic table in group 12, period 4. With an atomic number of 30, zinc is a naturally occurring element that has been utilized by humans for centuries due to its unique properties and versatility. From its earliest applications in ancient civilizations, such as in the production of brass, to its modern uses in fields like medicine, construction, and electronics, zinc has proven to be an essential component in various industries.
Zinc's importance extends beyond its practical applications, as it also plays a crucial role in biological systems. As an essential mineral, zinc is necessary for the proper functioning of the human body, with roles in immune function, wound healing, and protein synthesis. The element's ability to form complexes with various biomolecules has led to its incorporation in numerous enzymes, making it a vital component in maintaining overall health. With a recommended daily intake of 8-11 milligrams, zinc deficiency can lead to a range of health issues, including impaired growth and development, weakened immune systems, and increased susceptibility to infections.
Key Points
- Zinc is a transition metal with the symbol Zn and atomic number 30, located in group 12, period 4 of the periodic table.
- The element has been utilized by humans for centuries due to its unique properties, including its ability to form alloys and its high reactivity.
- Zinc plays a crucial role in biological systems, with essential functions in immune response, wound healing, and protein synthesis.
- The recommended daily intake of zinc is 8-11 milligrams, with deficiency leading to impaired growth, weakened immune systems, and increased infection susceptibility.
- Zinc's applications extend to various industries, including medicine, construction, and electronics, due to its versatility and unique properties.
Physical and Chemical Properties of Zinc
Zinc’s physical properties make it an attractive material for various applications. With a melting point of 419.53°C and a boiling point of 907°C, zinc exhibits a relatively low melting point compared to other transition metals. Its density of 7.14 g/cm³ is also noteworthy, as it is lower than that of many other metals. In terms of chemical properties, zinc is a highly reactive metal, readily forming compounds with other elements. Its most common oxidation state is +2, although it can also exhibit +1 and -2 oxidation states in certain compounds.
Zinc Alloys and Compounds
Zinc’s ability to form alloys with other metals has led to the creation of numerous materials with unique properties. Brass, an alloy of zinc and copper, is a prime example, with applications in musical instruments, hardware, and decorative items. Other notable alloys include zinc-nickel, zinc-tin, and zinc-lead, each with its own set of characteristics and uses. In addition to its alloys, zinc also forms a range of compounds, including zinc oxide, zinc sulfide, and zinc chloride, which have applications in fields such as cosmetics, pharmaceuticals, and textiles.
| Property | Value |
|---|---|
| Atomic Number | 30 |
| Atomic Mass | 65.38 u |
| Melting Point | 419.53°C |
| Boiling Point | 907°C |
| Density | 7.14 g/cm³ |

Biological Role of Zinc

Zinc’s role in biological systems is multifaceted and essential. As a cofactor for numerous enzymes, zinc plays a critical part in maintaining immune function, with deficiencies leading to impaired immune response and increased susceptibility to infections. The element is also involved in wound healing, with zinc-dependent enzymes contributing to the repair and regeneration of damaged tissue. Furthermore, zinc is necessary for protein synthesis, with the element acting as a cofactor for enzymes involved in transcription and translation.
Zinc Deficiency and Toxicity
While zinc is an essential mineral, both deficiency and toxicity can have significant health implications. Zinc deficiency, which can result from inadequate dietary intake, impaired absorption, or increased excretion, can lead to a range of health issues, including growth retardation, impaired immune function, and increased susceptibility to infections. On the other hand, zinc toxicity, which can occur through excessive dietary intake or exposure to zinc-containing materials, can cause symptoms such as nausea, vomiting, and diarrhea, as well as more severe health issues, including kidney damage and respiratory problems.
Zinc's importance extends beyond its biological role, with the element playing a critical part in various industries. From its use in galvanizing steel to protect against corrosion, to its application in batteries, zinc is a versatile material with a wide range of uses. As research continues to uncover the complexities of zinc's biological and chemical properties, it is likely that new applications and innovations will emerge, further solidifying the element's significance in modern society.
What is the primary biological function of zinc?
+Zinc plays a crucial role in immune function, wound healing, and protein synthesis, with the element acting as a cofactor for numerous enzymes involved in these processes.
What are the symptoms of zinc deficiency?
+Zinc deficiency can lead to a range of health issues, including impaired growth and development, weakened immune systems, and increased susceptibility to infections.
What are some common applications of zinc?
+Zinc is used in various industries, including medicine, construction, and electronics, due to its versatility and unique properties. Some common applications include galvanizing steel, producing batteries, and creating alloys such as brass.