Water, composed of two hydrogen atoms and one oxygen atom, is a molecule that has been extensively studied due to its unique properties and essential role in biological and chemical processes. One of the key characteristics of water is its polarity, which plays a crucial role in its chemical behavior and interactions with other molecules. The polarity of water arises from the difference in electronegativity between oxygen and hydrogen atoms, leading to a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This property is fundamental to understanding many of water's characteristics and its role in various biological, chemical, and physical phenomena.
Introduction to Polarity in Water Molecules
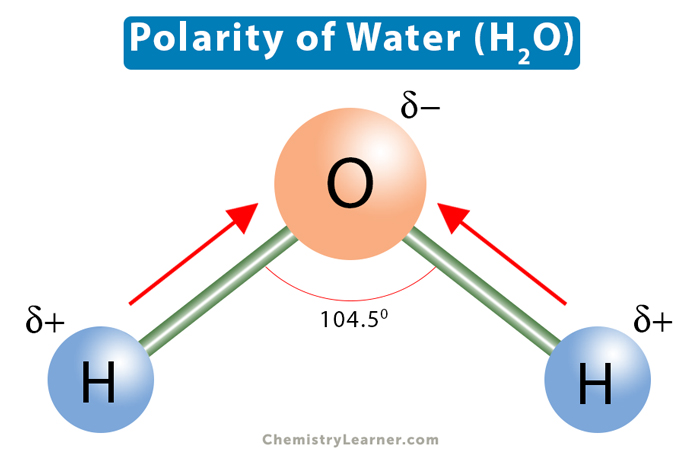
The concept of polarity in water molecules is rooted in the unequal sharing of electrons between the oxygen and hydrogen atoms. Oxygen, being more electronegative than hydrogen, pulls the shared electrons closer to itself, resulting in a partial negative charge (δ-) on the oxygen atom and a partial positive charge (δ+) on each of the hydrogen atoms. This dipole moment, where one end of the molecule is positively charged and the other end is negatively charged, is what makes water a polar molecule. Understanding the reasons behind water’s polarity is essential for grasping its solvent properties, its role in chemical reactions, and its biological significance.
Key Points
- Water's polarity is due to the unequal sharing of electrons between oxygen and hydrogen atoms.
- The difference in electronegativity between oxygen and hydrogen leads to partial positive and negative charges.
- Polarity is crucial for water's solvent properties and its role in biological processes.
- The molecular structure of water, with its bent or V-shape, contributes to its polarity.
- Hydrogen bonding, facilitated by polarity, is vital for the physical and chemical properties of water.
Reasons for Water’s Polarity

There are several reasons why water exhibits polarity, each contributing to its unique properties and behaviors. These reasons are interconnected and stem from the fundamental characteristics of the water molecule itself.
Electronegativity Difference
The primary reason for water’s polarity is the significant difference in electronegativity between oxygen (approximately 3.44 on the Pauling scale) and hydrogen (approximately 2.20 on the Pauling scale). This difference leads to an unequal sharing of electrons in the covalent bonds between oxygen and hydrogen, resulting in the oxygen atom having a partial negative charge and the hydrogen atoms having partial positive charges. This electronegativity difference is the foundation of water’s polarity and is responsible for its dipole moment.
Molecular Shape
The molecular shape of water, which is bent or V-shaped, also contributes to its polarity. This shape is due to the sp3 hybridization of the oxygen atom and the presence of two lone pairs of electrons on the oxygen. The bent shape ensures that the molecule has a net dipole moment, as the partial positive charges on the hydrogen atoms are not directly opposite the partial negative charge on the oxygen atom. This molecular geometry enhances the polarity of the water molecule, making it more effective as a solvent and facilitating its involvement in hydrogen bonding.
Hydrogen Bonding
Hydrogen bonding is a direct consequence of water’s polarity and is crucial for many of its physical and chemical properties. The partial positive charge on the hydrogen atoms of one water molecule can be attracted to the partial negative charge on the oxygen atom of another water molecule, forming a hydrogen bond. This type of bonding is relatively weak compared to covalent bonds but is strong enough to have a significant impact on the properties of water, such as its high boiling point, surface tension, and solvent capabilities.
Chemical Reactivity
The polarity of water also influences its chemical reactivity. As a polar solvent, water can dissolve a wide variety of substances, including salts, sugars, and other polar molecules. This is because the partial charges on the water molecules can interact with and stabilize the charged species (ions) that result from the dissolution of these substances. Additionally, water’s polarity allows it to participate in chemical reactions as a reactant, for example, in hydrolysis reactions where water is used to break chemical bonds.
Biological Significance
Finally, the polarity of water has profound implications for its biological significance. Water’s role as the medium of life is largely due to its polar nature, which allows it to dissolve nutrients, wastes, and other substances essential for living organisms. The polarity of water also influences the structure and function of biological molecules such as proteins and nucleic acids, as these molecules often rely on hydrogen bonding and other polar interactions to maintain their three-dimensional structures and perform their biological functions.
| Property | Value |
|---|---|
| Electronegativity of Oxygen | 3.44 |
| Electronegativity of Hydrogen | 2.20 |
| Dipole Moment of Water | 1.85 D |
| Boiling Point of Water | 100°C at 1 atm |
In conclusion, the polarity of water is a fundamental property that arises from the difference in electronegativity between oxygen and hydrogen atoms, the molecular shape of water, and the resulting dipole moment. This property is crucial for water's solvent capabilities, its role in chemical reactions, its biological significance, and its unique physical properties such as high boiling point and surface tension. The reasons for water's polarity are interconnected and underscore the importance of considering the molecular level when understanding the properties and behaviors of substances.
What is the primary reason for water’s polarity?
+The primary reason for water’s polarity is the difference in electronegativity between oxygen and hydrogen atoms, which leads to an unequal sharing of electrons and results in partial positive and negative charges within the molecule.
How does the molecular shape of water contribute to its polarity?
+The bent or V-shape of the water molecule, due to the sp3 hybridization of the oxygen atom and the presence of two lone pairs of electrons, ensures that the partial positive charges on the hydrogen atoms are not directly opposite the partial negative charge on the oxygen atom, thereby enhancing the molecule’s net dipole moment and polarity.
What role does hydrogen bonding play in the properties of water?
+Hydrogen bonding, facilitated by the polarity of water molecules, is crucial for many of water’s physical and chemical properties, including its high boiling point, surface tension, and solvent capabilities. Hydrogen bonds between water molecules allow them to interact and hold together, influencing the behavior of water in various contexts.



