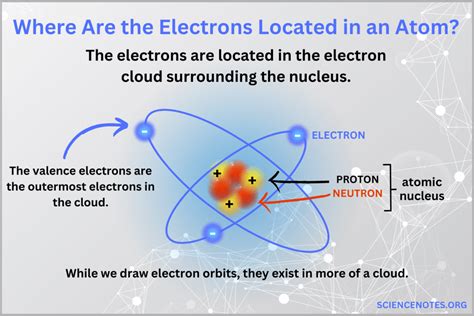
The discovery of electrons and their distribution within atomic orbitals has revolutionized our understanding of chemistry and physics. The concept of electrons occupying specific energy levels, or shells, around the nucleus of an atom has been a cornerstone of atomic theory since the early 20th century. In this article, we will delve into the intricacies of electron distribution in atomic orbitals, exploring the historical context, key principles, and modern applications of this fundamental concept.
Historically, the discovery of electrons is attributed to J.J. Thomson, who in 1897 conducted a series of experiments using cathode ray tubes. Thomson's work led to the development of the "plum pudding" model of the atom, which proposed that atoms consisted of a positively charged sphere with negatively charged electrons embedded within. Later, the Rutherford model, proposed by Ernest Rutherford in 1911, introduced the concept of a small, dense nucleus surrounded by electrons. However, it was not until the development of quantum mechanics in the 1920s that a comprehensive understanding of electron distribution in atomic orbitals began to take shape.
Key Points
- The electron configuration of an atom determines its chemical properties and reactivity.
- Atomic orbitals are mathematical descriptions of the regions around the nucleus where electrons are likely to be found.
- The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers.
- The Aufbau principle dictates that electrons occupy the lowest available energy levels.
- Hund's rule of maximum multiplicity states that electrons will occupy empty orbitals before pairing up in an orbital.
Electron Configuration and Atomic Orbitals
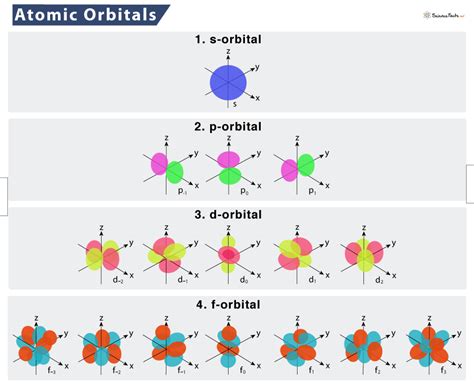
The arrangement of electrons in atomic orbitals is described by the electron configuration of an atom. Electron configuration is typically expressed using the noble gas core notation, where the inner electrons are represented by the symbol of the nearest noble gas, and the outer electrons are listed in terms of their orbital occupancy. For example, the electron configuration of carbon is 1s² 2s² 2p², indicating that the 1s and 2s orbitals are fully occupied, while the 2p orbitals are partially occupied.
Atomic orbitals are mathematical descriptions of the regions around the nucleus where electrons are likely to be found. These orbitals are characterized by a set of quantum numbers, including the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). The shape and orientation of atomic orbitals are determined by the values of these quantum numbers, with s orbitals being spherical, p orbitals being dumbbell-shaped, and d orbitals having a more complex, four-lobed shape.
Pauli Exclusion Principle and Electron Configuration
The Pauli Exclusion Principle, proposed by Wolfgang Pauli in 1925, states that no two electrons in an atom can have the same set of quantum numbers. This principle has a profound impact on electron configuration, as it dictates that each orbital can hold a maximum of two electrons, and that these electrons must have opposite spins. The Pauli Exclusion Principle is responsible for the observed chemical properties of elements, as it determines the number of electrons available for bonding and the resulting reactivity of an atom.
| Quantum Number | Description |
|---|---|
| Principal Quantum Number (n) | Determines the energy level of the orbital |
| Azimuthal Quantum Number (l) | Determines the shape of the orbital |
| Magnetic Quantum Number (m) | Determines the orientation of the orbital |
| Spin Quantum Number (s) | Determines the spin of the electron |
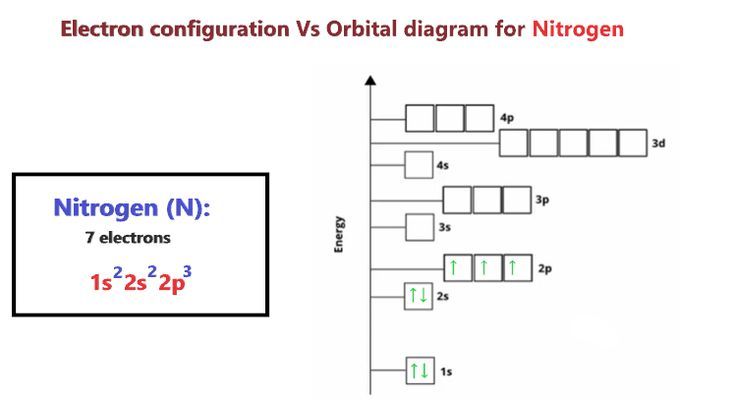
Aufbau Principle and Hund’s Rule
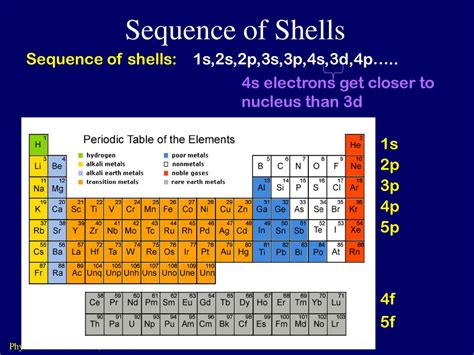
The Aufbau principle, also known as the building-up principle, dictates that electrons occupy the lowest available energy levels. This principle is responsible for the observed electron configuration of atoms, as electrons will always occupy the orbitals with the lowest energy before moving to higher energy levels. In conjunction with the Aufbau principle, Hund’s rule of maximum multiplicity states that electrons will occupy empty orbitals before pairing up in an orbital. This rule is responsible for the observed spin multiplicities of atoms, as electrons will always attempt to maximize their spin before pairing up.
In conclusion, the distribution of electrons in atomic orbitals is a complex and fascinating topic, with a rich history and profound implications for our understanding of chemistry and physics. By recognizing the principles that govern electron configuration, including the Pauli Exclusion Principle, the Aufbau principle, and Hund's rule, we can gain a deeper understanding of the behavior of electrons in atoms and the resulting chemical properties of elements.
What is the difference between an orbital and an electron shell?
+An orbital refers to a specific region around the nucleus where an electron is likely to be found, while an electron shell refers to a set of orbitals with the same principal quantum number.
How does the Pauli Exclusion Principle affect the electron configuration of an atom?
+The Pauli Exclusion Principle dictates that no two electrons in an atom can have the same set of quantum numbers, resulting in a maximum of two electrons per orbital and determining the observed chemical properties of elements.
What is the significance of the Aufbau principle in understanding electron configuration?
+The Aufbau principle dictates that electrons occupy the lowest available energy levels, resulting in the observed electron configuration of atoms and determining the chemical properties and reactivity of elements.