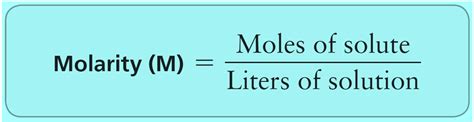Molality is a fundamental concept in chemistry, particularly in the field of physical chemistry and chemical engineering. It is a measure of the concentration of a solution, defined as the number of moles of solute per kilogram of solvent. Molality is an important property of solutions, as it affects the physical and chemical behavior of the solute and solvent. In this article, we will delve into the definition and explanation of molality, its significance, and its applications in various fields.
The concept of molality was first introduced by the French chemist Jean-Baptiste Dumas in the early 19th century. Dumas defined molality as the number of grams of solute per 1000 grams of solvent. However, this definition was later modified to moles of solute per kilogram of solvent, which is the currently accepted definition. Molality is denoted by the symbol 'm' and is expressed in units of moles per kilogram (mol/kg) or moles per liter (mol/L).
Key Points
- Molality is a measure of the concentration of a solution, defined as the number of moles of solute per kilogram of solvent.
- Molality is an important property of solutions, affecting the physical and chemical behavior of the solute and solvent.
- Molality is denoted by the symbol 'm' and is expressed in units of moles per kilogram (mol/kg) or moles per liter (mol/L).
- Molality is used in various fields, including chemistry, chemical engineering, and materials science.
- Molality is related to other concentration units, such as molarity, normality, and mass percentage.
Molality Formula and Calculation
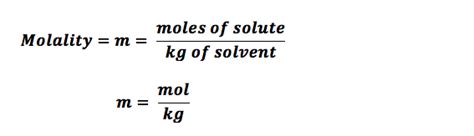
The molality of a solution can be calculated using the following formula: m = moles of solute / mass of solvent (in kg). The moles of solute can be calculated using the formula: moles = mass of solute / molar mass of solute. The mass of solvent can be measured using a balance or other weighing device. The molar mass of the solute can be obtained from a periodic table or other reference source.
For example, suppose we want to calculate the molality of a solution containing 20 grams of sodium chloride (NaCl) in 1000 grams of water. The molar mass of NaCl is 58.44 g/mol. First, we calculate the moles of NaCl: moles = 20 g / 58.44 g/mol = 0.342 mol. Then, we calculate the molality: m = 0.342 mol / 1 kg = 0.342 m. Therefore, the molality of the solution is 0.342 mol/kg.
Types of Molality
There are several types of molality, including:
- True molality: the number of moles of solute per kilogram of solvent, as defined above.
- Apparent molality: the number of moles of solute per kilogram of solvent, as measured using a hydrometer or other device.
- Effective molality: the number of moles of solute per kilogram of solvent, taking into account the effects of ionization and other chemical reactions.
| Property | True Molality | Apparent Molality | Effective Molality |
|---|---|---|---|
| Definition | moles of solute / kg of solvent | measured using hydrometer | takes into account ionization and reactions |
| Units | mol/kg | mol/kg | mol/kg |
| Application | chemistry, chemical engineering | chemical engineering, materials science | chemistry, chemical engineering, biology |

Molality vs. Molarity
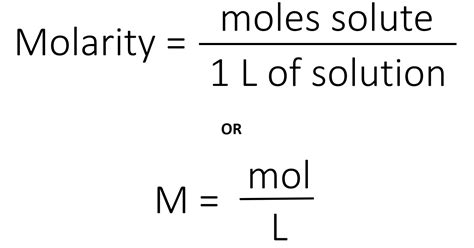
Molality and molarity are two related but distinct concepts in chemistry. Molarity is defined as the number of moles of solute per liter of solution, whereas molality is defined as the number of moles of solute per kilogram of solvent. While both units are used to express the concentration of a solution, they differ in their reference frame. Molarity is often used in laboratory settings, where the volume of the solution is easily measured. Molality, on the other hand, is more commonly used in industrial and engineering applications, where the mass of the solvent is more relevant.
The relationship between molality and molarity can be expressed using the following equation: m = (M \* ρ) / (1 + (M \* ρ)), where M is the molarity, ρ is the density of the solution, and m is the molality. This equation allows for the conversion between molality and molarity, taking into account the density of the solution.
Molality Applications
Molality has numerous applications in various fields, including:
- Chemistry: molality is used to express the concentration of solutions in chemical reactions, solubility, and other chemical processes.
- Chemical engineering: molality is used to design and optimize chemical processes, such as distillation, crystallization, and extraction.
- Materials science: molality is used to study the properties of materials, such as their solubility, diffusion, and reactivity.
- Biology: molality is used to study the behavior of biological molecules, such as proteins, nucleic acids, and cells, in various solutions.
What is the difference between molality and molarity?
+Molality is defined as the number of moles of solute per kilogram of solvent, whereas molarity is defined as the number of moles of solute per liter of solution. While both units are used to express the concentration of a solution, they differ in their reference frame.
How is molality calculated?
+Molality is calculated using the formula: m = moles of solute / mass of solvent (in kg). The moles of solute can be calculated using the formula: moles = mass of solute / molar mass of solute.
What are the applications of molality?
+Molality has numerous applications in various fields, including chemistry, chemical engineering, materials science, and biology. It is used to express the concentration of solutions, design and optimize chemical processes, study the properties of materials, and understand the behavior of biological molecules.
In conclusion, molality is a fundamental concept in chemistry and chemical engineering, used to express the concentration of solutions. Its applications are diverse, ranging from laboratory settings to industrial processes. By understanding the definition, calculation, and applications of molality, scientists and engineers can better design and optimize chemical processes, leading to more efficient and effective solutions.