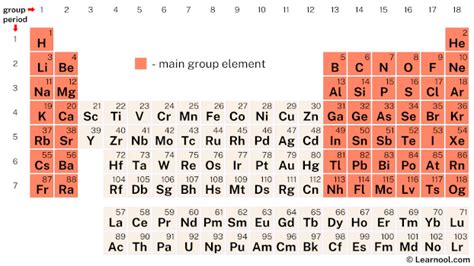
The concept of 5 main group elements is a fundamental aspect of chemistry, specifically within the context of the periodic table. The periodic table is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the atom's nucleus) and are grouped into rows called periods and columns called groups or families. The 5 main group elements are found in groups 1, 2, and 13 through 15 of the periodic table, excluding the transition metals and the noble gases.
Introduction to the 5 Main Group Elements
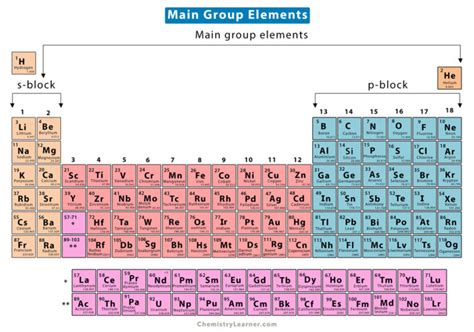
The 5 main group elements are typically categorized based on their electron configuration and chemical properties. These elements are: - Group 1: The Alkali Metals - Group 2: The Alkaline Earth Metals - Group 13: The Boron Group - Group 14: The Carbon Group - Group 15: The Nitrogen Group Each group exhibits unique properties due to the differences in their electron configurations, which influence their reactivity and chemical behavior.
Group 1: The Alkali Metals
Group 1 elements, also known as the alkali metals, include Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Francium (Fr). These metals are characterized by having one electron in their outermost shell, which they readily lose to form a positive ion. This property makes them highly reactive, especially with water and halogens. Their reactivity increases down the group due to the decrease in ionization energy.
Group 2: The Alkaline Earth Metals
Group 2 elements are known as the alkaline earth metals and include Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra). These elements have two electrons in their outermost shell and are less reactive than the alkali metals but more reactive than the transition metals and the rest of the periodic table. They also form positive ions but are more stable in their compounds due to the +2 charge, which allows them to achieve a noble gas configuration more easily.
Group 13: The Boron Group
Group 13 elements, or the boron group, include Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), and Thallium (Tl). These elements have three electrons in their outermost shell and exhibit a range of properties from non-metallic (boron) to metallic (the rest). Boron is a metalloid, and the rest are metals. Their reactivity and uses vary widely, with aluminum being one of the most widely used metals due to its strength, lightweight, and resistance to corrosion.
Group 14: The Carbon Group
Group 14 elements are known as the carbon group and include Carbon ©, Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb). Carbon is a non-metal, silicon and germanium are metalloids, and tin and lead are metals. This group shows a wide range of properties, from the organic compounds formed by carbon, which are the basis of life, to the inorganic compounds of the other elements, which have various industrial applications.
Group 15: The Nitrogen Group
Group 15 elements, or the nitrogen group, include Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi). Nitrogen and phosphorus are non-metals, arsenic and antimony are metalloids, and bismuth is a metal. These elements are crucial for life, with nitrogen being a key component of amino acids and phosphorus being essential for DNA and ATP. Their compounds have various applications, from fertilizers to semiconductors.
Key Points
- The 5 main group elements are categorized into groups 1, 2, and 13 through 15 of the periodic table.
- Each group exhibits unique chemical properties influenced by their electron configuration.
- Group 1 (Alkali Metals) and Group 2 (Alkaline Earth Metals) are highly reactive and form positive ions easily.
- Groups 13 (Boron Group), 14 (Carbon Group), and 15 (Nitrogen Group) show a wide range of properties from non-metallic to metallic and have various industrial and biological applications.
- Understanding the properties of these elements is crucial for advancements in chemistry, materials science, and biology.
| Group Number | Group Name | Examples of Elements |
|---|---|---|
| 1 | Alkali Metals | Lithium (Li), Sodium (Na), Potassium (K) |
| 2 | Alkaline Earth Metals | Beryllium (Be), Magnesium (Mg), Calcium (Ca) |
| 13 | Boron Group | Boron (B), Aluminum (Al), Gallium (Ga) |
| 14 | Carbon Group | Carbon (C), Silicon (Si), Germanium (Ge) |
| 15 | Nitrogen Group | Nitrogen (N), Phosphorus (P), Arsenic (As) |
What are the 5 main group elements in the periodic table?
+The 5 main group elements are categorized into groups 1 (Alkali Metals), 2 (Alkaline Earth Metals), and 13 (Boron Group), 14 (Carbon Group), and 15 (Nitrogen Group) of the periodic table.
What determines the chemical properties of the 5 main group elements?
+The chemical properties of the 5 main group elements are primarily determined by their electron configuration, especially the number of electrons in the outermost shell.
Why are the alkali metals (Group 1) highly reactive?
+The alkali metals are highly reactive because they have one electron in their outermost shell, which they can easily lose to form a positive ion, thus achieving a noble gas configuration.
What are some applications of the elements in Group 14 (Carbon Group)?
+Group 14 elements have a wide range of applications. Carbon is the basis of all life and is used in numerous organic compounds. Silicon is crucial for the production of semiconductors and computer chips. Tin is used as a protective coating for steel, and lead has applications in batteries and radiation shielding.
How do the properties of elements in the same group change as you move down the group?
+As you move down a group, the elements tend to become more metallic and their reactivity increases due to the decrease in ionization energy. This is because the outermost electron is farther away from the nucleus, making it easier to remove.