The concept of what an atom looks like has undergone significant transformations since the early days of atomic theory. Initially, atoms were thought to be indivisible, solid spheres, a notion that has been vastly refined and expanded upon through the advancements in physics and chemistry. The modern understanding of atomic structure reveals a complex, dynamic system comprising protons, neutrons, and electrons, each playing a crucial role in defining the atom's properties and behavior.
Historical Perspective on Atomic Structure
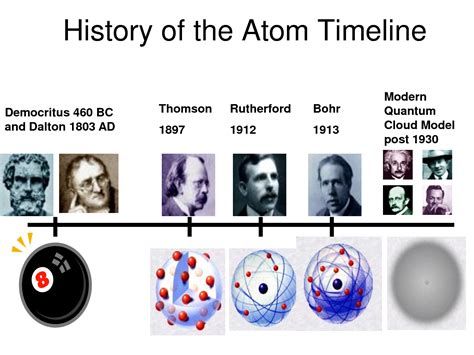
The earliest models of the atom, such as the “plum pudding” model proposed by J.J. Thomson, depicted atoms as positively charged spheres with negatively charged electrons embedded within. However, with the discovery of the nucleus by Ernest Rutherford in 1911, our understanding began to shift towards a more nuanced view. Rutherford’s gold foil experiment revealed that atoms have a small, dense nucleus at their center, surrounded by electrons. This marked the beginning of the nuclear model of the atom, which has been further refined by subsequent discoveries.
The Nuclear Model and Electron Orbitals
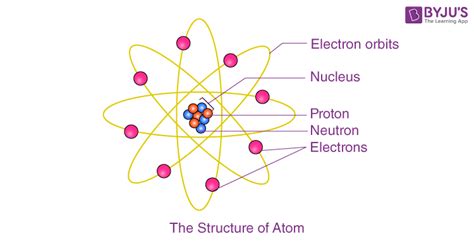
The nuclear model, as developed by Niels Bohr, introduces the concept of electron orbitals or shells around the nucleus. According to this model, electrons occupy specific energy levels or shells around the nucleus, with each shell capable of holding a certain number of electrons. The arrangement of electrons in these shells determines the chemical properties of an element. The nucleus itself is composed of protons and neutrons, with the number of protons defining the element (atomic number) and the sum of protons and neutrons determining the atom’s mass (atomic mass number).
| Atomic Component | Description |
|---|---|
| Protons | Positively charged particles found in the nucleus, determining the element's identity. |
| Neutrons | Particles with no charge, found in the nucleus, contributing to the atom's mass. |
| Electrons | Negatively charged particles orbiting the nucleus, influencing chemical properties. |
Modern Understanding: Quantum Mechanics and Atomic Models
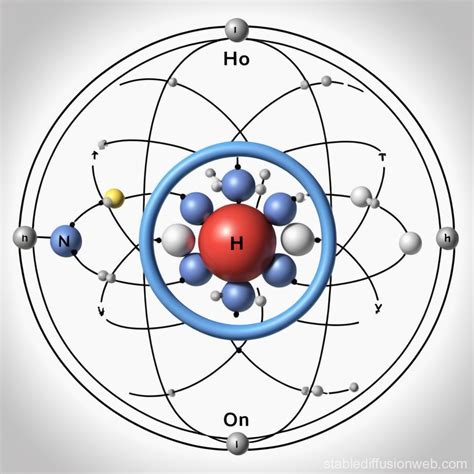
The advent of quantum mechanics has further refined our understanding of atomic structure, introducing the concept of wave functions and probability distributions for electron locations. According to quantum mechanics, electrons do not occupy definite paths or orbits but instead exist in cloud-like regions around the nucleus, known as atomic orbitals. This probabilistic approach allows for the prediction of electron behaviors and the calculation of various atomic properties with high accuracy.
Visualizing Atoms: Challenges and Advances
Visualizing the atom directly is a significant challenge due to its incredibly small size. However, through various techniques such as scanning tunneling microscopy (STM) and transmission electron microscopy (TEM), scientists can indirectly observe the structure of atoms and molecules. These methods have enabled the creation of detailed images of atomic surfaces and the manipulation of individual atoms, providing insights into atomic arrangements and behaviors at the nanoscale.
Key Points
- The modern atomic model consists of a nucleus surrounded by electrons in orbitals, as described by quantum mechanics.
- The arrangement of electrons in these orbitals determines the chemical properties of an element.
- Direct visualization of atoms is challenging, but techniques like STM and TEM allow for indirect observation and manipulation at the nanoscale.
- Understanding atomic structure is crucial for explaining chemical reactions, material properties, and biological processes.
- Continuous advancements in physics and chemistry have refined our knowledge of atomic structure, from early models to the current quantum mechanical understanding.
In conclusion, the question of what an atom looks like has evolved significantly over time, from the simplistic models of the past to the complex, dynamic systems understood today. The integration of quantum mechanics and the development of advanced imaging techniques have provided unparalleled insights into the structure and behavior of atoms, underpinning our understanding of matter at its most fundamental level.
What is the basic structure of an atom?
+The basic structure of an atom includes a nucleus composed of protons and neutrons, surrounded by electrons in specific energy levels or orbitals.
How do electrons arrange themselves around the nucleus?
+Electrons arrange themselves in specific orbitals or shells around the nucleus, with each shell having a capacity for a certain number of electrons. The arrangement of electrons in these shells determines the chemical properties of an element.
Why is it challenging to visualize atoms directly?
+Visualizing atoms directly is challenging due to their incredibly small size. However, techniques like scanning tunneling microscopy (STM) and transmission electron microscopy (TEM) allow for indirect observation and manipulation of atoms at the nanoscale.



