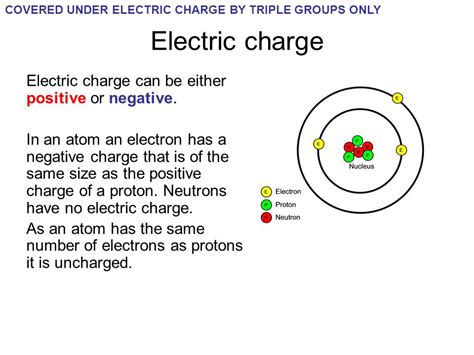
The electron, a fundamental particle in physics, carries a negative charge, which is a fundamental property of matter. This negative charge is a cornerstone of our understanding of the behavior of electrons in various environments, from the atomic scale to complex electronic devices. The discovery of the electron's negative charge is attributed to the work of J.J. Thomson in the late 19th century, who, through his experiments with cathode rays, demonstrated that these rays were composed of negatively charged particles. This finding revolutionized the field of physics, paving the way for the development of quantum mechanics and the understanding of the atomic structure.
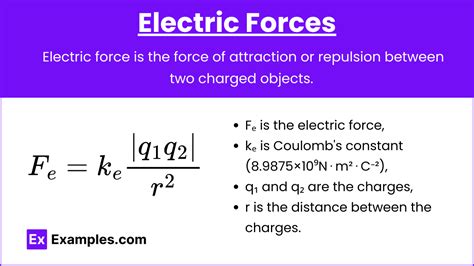
The negative charge of an electron is denoted by the symbol e^- and has a magnitude of approximately 1.602 x 10^-19 Coulombs. This charge, though small in magnitude, plays a crucial role in the electrostatic forces that govern the interactions between charged particles. The electrostatic force between two charges is described by Coulomb's Law, which states that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. In the context of electrons, this means that two electrons will repel each other due to their like charges, while an electron and a proton (which carries a positive charge) will attract each other.
Key Points
- The electron has a negative charge, denoted as e^-, with a magnitude of approximately 1.602 x 10^-19 Coulombs.
- The discovery of the electron's negative charge by J.J. Thomson was a pivotal moment in the history of physics.
- Coulomb's Law describes the electrostatic forces between charged particles, including electrons.
- The negative charge of electrons plays a crucial role in their interactions and behaviors in various physical systems.
- Understanding electron charge is essential for the development of electronic devices and technologies.
Electron Charge and Atomic Structure
The negative charge of electrons is a critical component of the atomic structure. Atoms, the basic building blocks of matter, consist of a nucleus surrounded by electrons. The nucleus contains protons, which have a positive charge, and neutrons, which are neutral. The number of protons in the nucleus defines the element of an atom, while the number of electrons, which must balance the positive charge of the protons to maintain neutrality, determines the chemical properties of an element. The arrangement of electrons around the nucleus follows specific patterns, described by electron shells or orbitals, which are crucial for understanding chemical bonding and reactions.
Electron Configuration and Chemical Properties
The configuration of electrons in an atom, particularly the outermost electrons, is responsible for its chemical properties. These outer electrons, known as valence electrons, participate in chemical bonding with other atoms. The desire of atoms to achieve a stable electronic configuration, similar to that of the noble gases, drives chemical reactions. For instance, atoms with few electrons in their outer shell tend to lose them to form positive ions (cations), while those with nearly full outer shells tend to gain electrons to form negative ions (anions). The understanding of electron configuration and its influence on chemical properties is fundamental in chemistry and materials science.
| Electron Property | Value |
|---|---|
| Charge | -1.602 x 10^-19 C |
| Mass | 9.109 x 10^-31 kg |
| Spin | 1/2 |
Applications of Electron Charge
The negative charge of electrons has numerous practical applications in electronics and technology. Electronic devices, such as transistors, diodes, and integrated circuits, rely on the controlled flow of electrons to operate. The field of electronics has revolutionized modern life, enabling the development of computers, smartphones, televisions, and countless other devices that rely on the manipulation of electron flow. Furthermore, the understanding of electron charge is crucial in the development of new materials and technologies, including solar cells, where the flow of electrons is harnessed to generate electricity from sunlight.
Future Perspectives and Research Directions
Research into the properties and behaviors of electrons continues to advance our understanding of the physical world and drives innovation. Quantum computing, for example, relies on the unique properties of electrons, such as spin and superposition, to perform calculations that are beyond the capabilities of classical computers. Additionally, the study of electron transport in novel materials, like graphene and topological insulators, is opening new avenues for the development of faster, smaller, and more efficient electronic devices. As our understanding of electron charge and its applications deepens, we can expect significant advancements in technology and our ability to manipulate matter at the atomic and subatomic level.
What is the magnitude of the electron's charge?
+The magnitude of the electron's charge is approximately 1.602 x 10^-19 Coulombs.
Why is the negative charge of electrons important in chemistry?
+The negative charge of electrons is crucial in chemistry because it determines the chemical properties of elements and how they react with each other to form compounds.
What are some practical applications of the electron's negative charge?
+The electron's negative charge has numerous practical applications in electronics, including the development of transistors, diodes, and integrated circuits, which are essential components of modern electronic devices.
Meta Description: Discover the significance of the electron’s negative charge and its implications for our understanding of physics, chemistry, and the development of electronic devices. Explore how this fundamental property influences atomic structure, chemical reactions, and technological advancements.