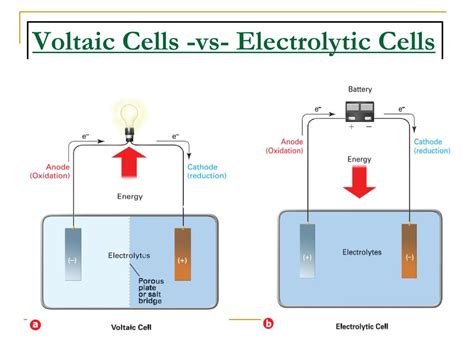
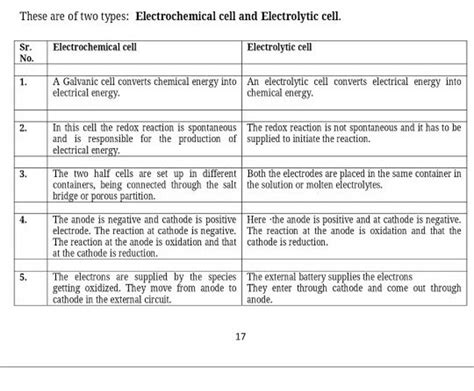
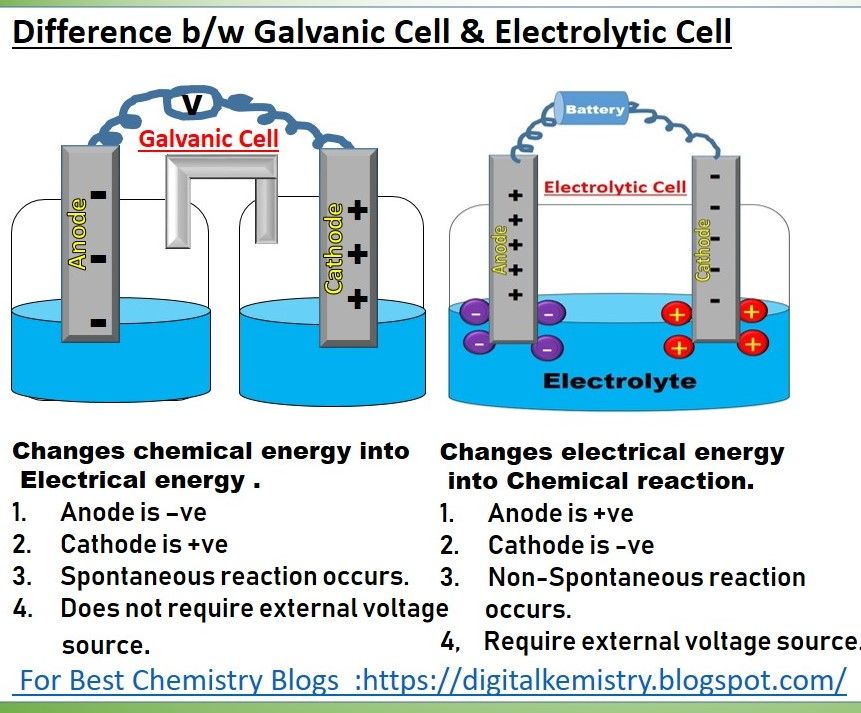
The study of electrochemistry is rooted in the understanding of two fundamental concepts: the voltaic cell and the electrolytic cell. These cells are the backbone of electrochemical reactions, which are crucial in various industrial, commercial, and research applications. A voltaic cell, also known as a galvanic cell, is an electrochemical cell that generates an electric current from a spontaneous redox reaction. On the other hand, an electrolytic cell uses an external electrical current to drive a non-spontaneous redox reaction, often for the purpose of depositing a substance or causing a chemical change. The distinction between these two types of cells lies in the direction of electron flow and the spontaneity of the reactions they facilitate.
Key Points
- The voltaic cell generates an electric current from a spontaneous redox reaction, converting chemical energy into electrical energy.
- The electrolytic cell, in contrast, requires an external electrical current to drive a non-spontaneous redox reaction, often for the purpose of electrolysis or electroplating.
- The direction of electron flow is from the anode to the cathode in both cells, but in a voltaic cell, this flow occurs naturally, while in an electrolytic cell, it is forced by an external power source.
- The spontaneity of the reaction is a critical difference; voltaic cells operate under spontaneous conditions, whereas electrolytic cells require energy input to proceed.
- Applications of voltaic cells include batteries, such as those used in vehicles and portable electronics, while electrolytic cells are used in electroplating, refining metals, and the production of chlorine and sodium hydroxide.
Principle of Operation: Voltaic Cell
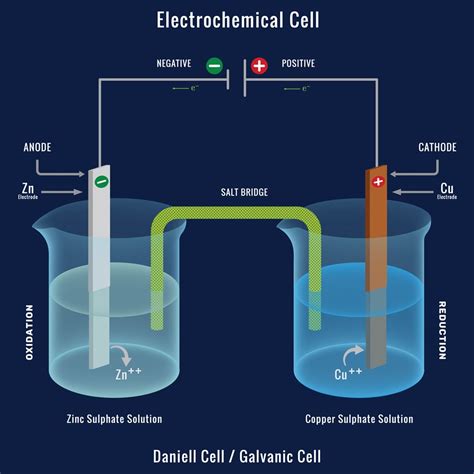
A voltaic cell consists of two half-cells, each containing an electrode and an electrolyte. The electrodes are typically made of different materials, with one acting as the anode (where oxidation occurs) and the other as the cathode (where reduction occurs). The electrolytes can be in the form of solutions or solids, facilitating the flow of ions between the electrodes. When the electrodes are connected through an external circuit, electrons flow from the anode to the cathode, generating an electric current. This process is spontaneous and is the basis for batteries and other electrochemical power sources. The voltaic cell’s operation can be represented by the reaction: Zn (s) + Cu²⁺ (aq) → Zn²⁺ (aq) + Cu (s), where zinc is oxidized at the anode, and copper ions are reduced at the cathode.
Electrochemical Reactions in Voltaic Cells
The electrochemical reactions in a voltaic cell are characterized by the transfer of electrons from one species to another, resulting in a change in oxidation state. The anode, where oxidation occurs, is the site of electron loss, while the cathode, where reduction occurs, is the site of electron gain. The potential difference between the two electrodes, known as the electromotive force (EMF), drives the reaction and determines the cell’s voltage. The standard electrode potentials of the half-reactions involved can be used to predict the overall cell potential, providing insight into the spontaneity and feasibility of the reaction.
| Component | Voltaic Cell | Electrolytic Cell |
|---|---|---|
| Reaction Type | Spontaneous | Non-spontaneous |
| Electron Flow | Natural, from anode to cathode | Forced by external current |
| Energy Requirement | No external energy required | External electrical energy required |
| Applications | Batteries, power generation | Electroplating, metal refining, chemical synthesis |
Principle of Operation: Electrolytic Cell
An electrolytic cell, on the other hand, operates by using an external electrical current to drive a non-spontaneous redox reaction. This process is the reverse of what occurs in a voltaic cell and requires an energy input to force the reaction. The electrolytic cell is commonly used for electroplating, where a metal ion in solution is reduced to its elemental form and deposited onto a surface, and for the refining of metals, where impure metals are purified through electrolysis. The electrolytic cell’s operation can be represented by the reaction: 2H₂O (l) → 2H₂ (g) + O₂ (g), where water is split into hydrogen and oxygen gases under the influence of an external electrical current.
Applications of Electrolytic Cells
The applications of electrolytic cells are diverse and reflect the cell’s ability to drive non-spontaneous reactions. In electroplating, an electrolytic cell is used to deposit a thin layer of a material, often a metal, onto the surface of another material. This process is used to modify the surface properties of the material, such as its corrosion resistance, wear resistance, or aesthetic appearance. In the production of chlorine and sodium hydroxide, electrolytic cells are used to decompose sodium chloride (common salt) into these two industrially important chemicals. The refining of metals, such as copper and zinc, also relies on electrolytic cells to produce high-purity metals from impure ores or scrap metals.
What is the primary difference between a voltaic cell and an electrolytic cell?
+The primary difference lies in the direction of electron flow and the spontaneity of the reactions. A voltaic cell generates an electric current from a spontaneous redox reaction, while an electrolytic cell requires an external electrical current to drive a non-spontaneous redox reaction.
What are some common applications of voltaic cells?
+Voltaic cells are commonly used in batteries, such as those used in vehicles and portable electronics, providing a source of electrical energy through spontaneous redox reactions.
How does an electrolytic cell differ from a voltaic cell in terms of energy requirement?
+An electrolytic cell requires an external electrical energy input to drive the non-spontaneous redox reaction, whereas a voltaic cell does not require any external energy input, as the reaction is spontaneous.
In conclusion, the distinction between voltaic and electrolytic cells is fundamental to understanding electrochemical principles and their applications. While both types of cells facilitate redox reactions, the spontaneity of the reaction, the direction of electron flow, and the energy requirements are key differences that define their operation and utility. As technology continues to evolve, the importance of these cells in energy storage, conversion, and various industrial processes will only continue to grow, underscoring the need for a deep understanding of their principles and applications.