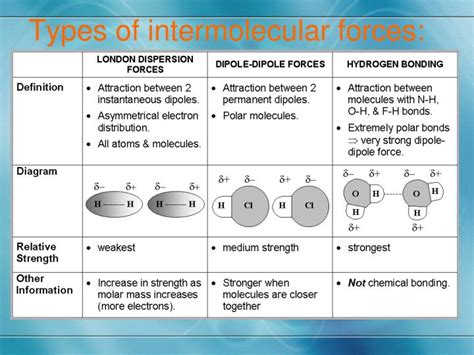
The realm of intermolecular forces is a complex and fascinating area of study in chemistry, playing a crucial role in determining the physical properties of substances. These forces are responsible for the interactions between molecules, influencing everything from the boiling and melting points of a substance to its viscosity and surface tension. In essence, understanding intermolecular forces is key to grasping why substances behave the way they do under various conditions. There are several types of intermolecular forces, each with its unique characteristics and influences on the behavior of molecules.
Key Points
- Intermolecular forces are interactions between molecules that are not as strong as the chemical bonds within the molecules themselves.
- There are several types of intermolecular forces, including London dispersion forces, dipole-dipole forces, and hydrogen bonding.
- London dispersion forces are present in all molecules, regardless of their polarity, and are responsible for the attraction between non-polar molecules.
- Dipole-dipole forces occur between polar molecules and are stronger than London dispersion forces but weaker than hydrogen bonds.
- Hydrogen bonding is a specific type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule or within the same molecule if it's sufficiently distant.
- The strength and type of intermolecular forces present in a substance determine its physical properties, such as melting and boiling points, viscosity, and solubility.
London Dispersion Forces
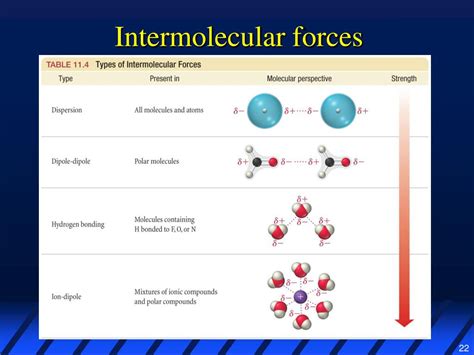
London dispersion forces, also known as van der Waals forces, are the weakest of the intermolecular forces and are present in all molecules, regardless of whether they are polar or non-polar. These forces arise due to temporary dipoles that form in atoms or molecules as a result of the movement of electrons around the nucleus. Even in non-polar molecules, the electrons are not always evenly distributed, leading to temporary dipoles. When two non-polar molecules are near each other, the temporary dipoles in one molecule can induce dipoles in the other, leading to a weak attractive force between the molecules. The strength of London dispersion forces depends on the size of the molecule (larger molecules tend to have stronger dispersion forces) and the polarity of the molecule, but they are generally weaker than other types of intermolecular forces.
Factors Influencing London Dispersion Forces
The strength of London dispersion forces is influenced by the molecular weight and the shape of the molecule. Generally, as the molecular weight increases, the strength of the London dispersion forces also increases because larger molecules have more electrons, which can lead to a greater temporary dipole moment. Additionally, the shape of the molecule can affect how closely molecules can approach each other, thus influencing the strength of the dispersion forces. Branched molecules, for example, may have weaker dispersion forces than linear molecules of similar molecular weight because their branched shape prevents them from packing as closely together.
| Type of Intermolecular Force | Strength | Conditions for Occurrence |
|---|---|---|
| London Dispersion Forces | Weakest | Present in all molecules |
| Dipole-Dipole Forces | Medium | Between polar molecules |
| Hydrogen Bonding | Strongest | Between molecules with a hydrogen atom bonded to a highly electronegative atom |
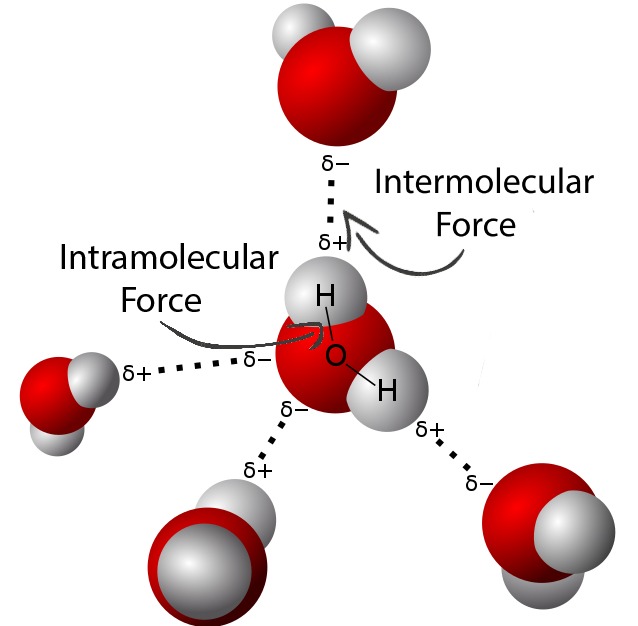
Dipole-Dipole Forces

Dipole-dipole forces are stronger than London dispersion forces and occur between molecules that are polar, meaning they have a permanent electric dipole moment. This polarity arises from a difference in electronegativity between the atoms in a bond, leading to a partial positive charge on one part of the molecule and a partial negative charge on another part. When two polar molecules are near each other, the positive end of one molecule is attracted to the negative end of the other, resulting in a dipole-dipole interaction. The strength of dipole-dipole forces depends on the magnitude of the dipole moments of the molecules involved and the distance between them.
Examples of Dipole-Dipole Forces
Dipole-dipole forces are commonly observed in substances like water (H2O) and ammonia (NH3). In water, the oxygen atom is more electronegative than the hydrogen atoms, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens. This polarity leads to significant dipole-dipole interactions between water molecules, which are crucial for many of water’s unique properties, such as its high boiling point compared to other substances of similar molecular weight.
Hydrogen Bonding
Hydrogen bonding is a specific and relatively strong type of dipole-dipole interaction that occurs when a hydrogen atom, which is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine), is attracted to another electronegative atom in a different molecule or within the same molecule if it is sufficiently distant. Hydrogen bonds are stronger than London dispersion forces and most dipole-dipole forces, making them crucial in determining the structure and properties of substances, especially in biological systems. For example, hydrogen bonding is responsible for the structure of DNA (the double helix model) and the secondary, tertiary, and quaternary structures of proteins.
Importance of Hydrogen Bonding in Biological Systems
In biological systems, hydrogen bonding plays a pivotal role in the recognition and binding of molecules, such as the binding of enzymes to their substrates, the interaction between drugs and their receptors, and the folding of proteins into their functional shapes. The specificity and strength of hydrogen bonds allow for precise interactions that are essential for the proper functioning of biological molecules and processes.
What are the main types of intermolecular forces?
+The main types of intermolecular forces are London dispersion forces, dipole-dipole forces, and hydrogen bonding. Each type of force has distinct characteristics and plays a different role in determining the physical properties of substances.
How do intermolecular forces affect the physical properties of substances?
+Intermolecular forces significantly affect the physical properties of substances, including their melting and boiling points, viscosity, surface tension, and solubility. The strength and type of intermolecular forces present in a substance determine these properties, with stronger forces generally leading to higher melting and boiling points, higher viscosity, and lower vapor pressure.
What is the role of hydrogen bonding in biological systems?
+Hydrogen bonding plays a crucial role in biological systems, including the structure of DNA and proteins, the recognition and binding of molecules, and the proper functioning of enzymes and other biological molecules. The specificity and strength of hydrogen bonds are essential for these processes, allowing for precise interactions that are vital for life.
In conclusion, intermolecular forces are a critical aspect of chemistry, influencing the behavior of substances in various environments. Understanding the different types of intermolecular forces, including London dispersion forces, dipole-dipole forces, and hydrogen bonding, is essential for predicting the physical properties of substances and their interactions in chemical and biological systems. The nuances of these forces, including their strengths, the conditions under which they occur, and their roles in biological processes, underscore the complexity and beauty of molecular interactions.