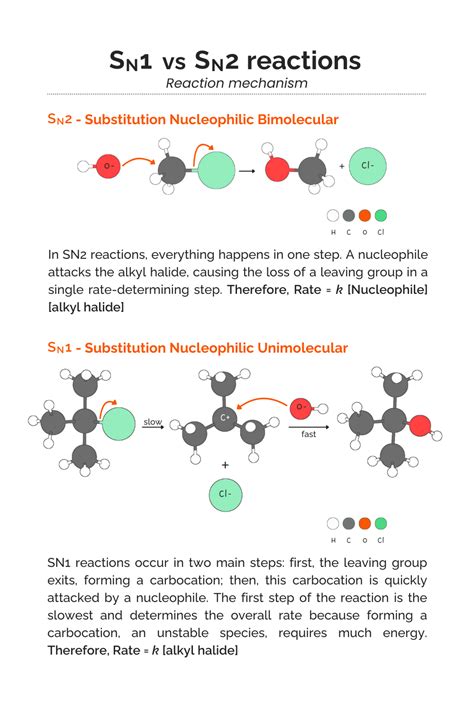
The realm of organic chemistry is replete with complex reactions, each with its unique characteristics and mechanisms. Among these, the SN1 and SN2 reactions stand out as fundamental processes in the study of nucleophilic substitution. These reactions are pivotal in understanding how molecules interact and transform, and their differences are crucial for chemists to predict and manipulate the outcomes of various chemical syntheses. The SN1 and SN2 reactions are named based on their kinetic orders, with SN1 being unimolecular and SN2 being bimolecular, reflecting the number of molecules involved in the rate-determining step of each reaction.
Key Points
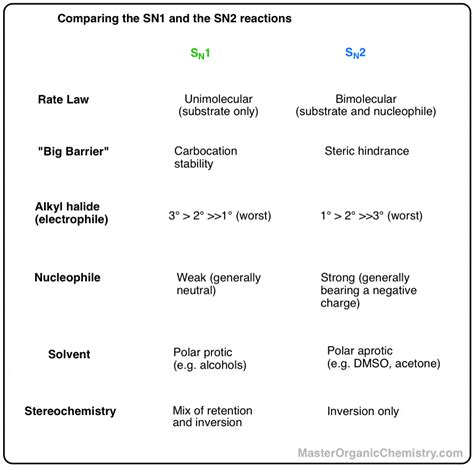
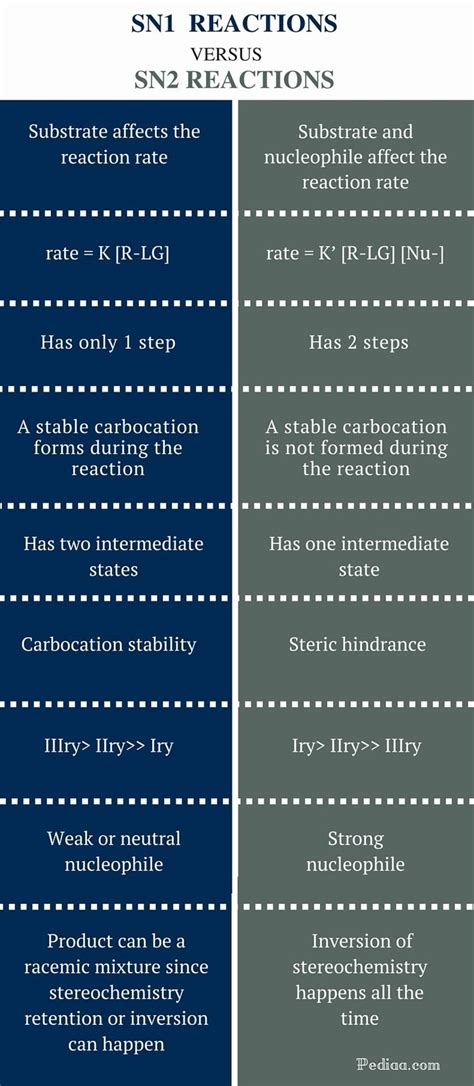
- The SN1 reaction is a two-step process involving the formation of a carbocation intermediate, which then reacts with a nucleophile.
- The SN2 reaction is a concerted, one-step process where the nucleophile attacks the carbon atom bearing the leaving group from the backside, resulting in a stereoinversion.
- SN1 reactions are typically favored in secondary substrates and are highly sensitive to solvent effects, whereas SN2 reactions are more common with primary substrates and are less sensitive to the solvent.
- The stereochemistry of the product differs significantly between SN1 and SN2 reactions, with SN1 reactions often leading to racemization and SN2 reactions resulting in inversion of configuration.
- Understanding the differences between SN1 and SN2 reactions is crucial for the design and optimization of synthetic routes in organic chemistry.
SN1 Reaction Mechanism and Characteristics
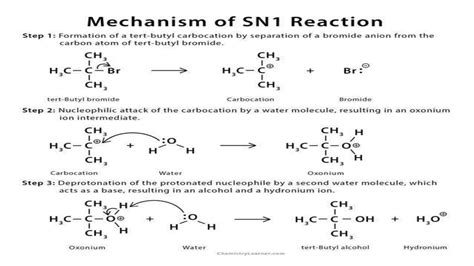
The SN1 reaction mechanism involves two distinct steps. The first step is the formation of a carbocation intermediate, which occurs when the leaving group departs from the substrate, creating a positively charged carbon atom. This step is the rate-determining step of the SN1 reaction and is unimolecular, meaning the rate of reaction depends solely on the concentration of the substrate. The second step involves the nucleophile attacking the carbocation to form the product. This step is typically fast and does not determine the overall rate of the reaction. The SN1 reaction is characterized by its sensitivity to solvent effects, with polar protic solvents facilitating the formation of the carbocation by stabilizing the transition state. Additionally, the presence of a good leaving group is essential for the SN1 reaction to proceed efficiently.
Factors Influencing SN1 Reactions
Several factors can influence the likelihood and efficiency of an SN1 reaction. The nature of the substrate, particularly whether it is primary, secondary, or tertiary, plays a significant role. Secondary substrates are more prone to undergo SN1 reactions due to the relative stability of the secondary carbocation intermediate. Furthermore, the solvent in which the reaction is carried out can significantly impact the reaction rate, with polar protic solvents like methanol or water being more conducive to SN1 reactions than non-polar aprotic solvents like dichloromethane or tetrahydrofuran.
| Substrate Type | Relative Reactivity in SN1 |
|---|---|
| Tertiary | High |
| Secondary | Moderate |
| Primary | Low |
SN2 Reaction Mechanism and Characteristics
In contrast to the SN1 reaction, the SN2 reaction is a concerted, one-step process. It involves the nucleophile approaching the carbon atom bearing the leaving group from the backside, leading to the simultaneous departure of the leaving group and the formation of the new bond between the nucleophile and the carbon atom. This backside attack results in an inversion of configuration at the affected carbon atom. The SN2 reaction is bimolecular, meaning the rate of reaction depends on the concentrations of both the substrate and the nucleophile. SN2 reactions are less sensitive to solvent effects compared to SN1 reactions but are more sensitive to steric effects due to the requirement for backside attack.
Factors Influencing SN2 Reactions
The SN2 reaction is favored in primary substrates due to less steric hindrance, which allows for easier backside attack by the nucleophile. The nature of the nucleophile also plays a crucial role, with stronger nucleophiles leading to faster reaction rates. The solvent can influence the reaction by affecting the nucleophile’s strength, with polar aprotic solvents often enhancing the nucleophilicity of anions by preventing their solvation and thus making them more reactive.
| Nucleophile Strength | Relative Reactivity in SN2 |
|---|---|
| Strong Nucleophile | High |
| Weak Nucleophile | Low |
In conclusion, understanding the differences between SN1 and SN2 reactions is crucial for predicting and controlling the outcomes of nucleophilic substitution reactions. By recognizing the factors that influence these reactions, such as substrate type, solvent effects, and nucleophile strength, chemists can design and optimize synthetic routes to achieve specific chemical transformations efficiently and effectively.
What is the primary difference between SN1 and SN2 reactions in terms of their mechanisms?
+The primary difference lies in their mechanisms; SN1 reactions are two-step processes involving a carbocation intermediate, while SN2 reactions are one-step, concerted processes involving backside attack by the nucleophile.
How do solvent effects differ between SN1 and SN2 reactions?
+SN1 reactions are highly sensitive to solvent effects, with polar protic solvents facilitating the reaction by stabilizing the carbocation intermediate. In contrast, SN2 reactions are less sensitive to solvent effects but can be influenced by the solvent’s ability to solvate the nucleophile.
What is the stereochemical outcome of SN1 vs. SN2 reactions?
+SN1 reactions often lead to racemization due to the planar nature of the carbocation intermediate, while SN2 reactions result in a clean inversion of configuration due to the backside attack by the nucleophile.