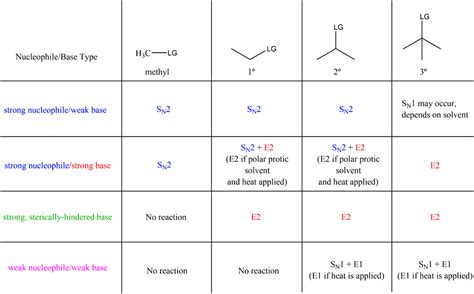
The realm of organic chemistry is replete with complex reactions, each governed by its own set of rules and mechanisms. Among these, substitution and elimination reactions are fundamental, providing the basis for understanding a wide array of chemical transformations. Specifically, SN1, SN2, E1, and E2 reactions are cornerstone concepts in organic chemistry, representing different pathways through which molecules can undergo substitution or elimination. Understanding these reactions is crucial for any student or practitioner of organic chemistry, as they form the basis of numerous synthetic strategies and mechanistic explanations.
Introduction to Substitution and Elimination Reactions
Substitution reactions involve the replacement of a leaving group in a molecule with another group, while elimination reactions involve the removal of a leaving group and a beta-hydrogen, resulting in the formation of a new bond. The distinction between these reactions lies in their mechanisms, which are influenced by factors such as the nature of the substrate, the leaving group, the solvent, and the reaction conditions. SN1 and SN2 are types of nucleophilic substitution reactions, differing in their mechanisms and the conditions under which they occur. Similarly, E1 and E2 are types of elimination reactions, also distinguished by their mechanisms and reaction conditions.
SN1 Reactions: Unimolecular Nucleophilic Substitution
SN1 reactions are characterized by a unimolecular mechanism, meaning the rate-determining step involves only one molecule. This type of reaction typically occurs with tertiary substrates, which have three alkyl groups attached to the carbon bearing the leaving group. The mechanism involves the initial formation of a carbocation intermediate, which then reacts with a nucleophile to form the product. The SN1 mechanism is consistent with a two-step process: the first step is the dissociation of the leaving group to form a carbocation, and the second step is the nucleophilic attack on the carbocation. This reaction is favored in polar protic solvents, which can stabilize the carbocation intermediate.
| Reaction Characteristics | SN1 |
|---|---|
| Substrate | Tertiary |
| Leaving Group | Good leaving group |
| Solvent | Polar protic |
| Rate-Determining Step | Formation of carbocation |
SN2 Reactions: Bimolecular Nucleophilic Substitution
SN2 reactions, on the other hand, follow a bimolecular mechanism, where the rate-determining step involves two molecules: the substrate and the nucleophile. This reaction typically occurs with primary substrates, which have one alkyl group attached to the carbon bearing the leaving group. The SN2 mechanism is a concerted process, involving the simultaneous attack of the nucleophile and the departure of the leaving group, resulting in a stereochemical inversion at the reaction site. SN2 reactions are favored in polar aprotic solvents, which do not interfere with the nucleophile’s approach to the substrate.
| Reaction Characteristics | SN2 |
|---|---|
| Substrate | Primary |
| Leaving Group | Good leaving group |
| Solvent | Polar aprotic |
| Rate-Determining Step | Nucleophilic attack |
E1 Reactions: Unimolecular Elimination
E1 reactions are unimolecular elimination reactions, meaning the rate-determining step involves only one molecule. Similar to SN1 reactions, E1 reactions also involve the formation of a carbocation intermediate. However, instead of being attacked by a nucleophile, a beta-hydrogen is eliminated from the carbocation, resulting in the formation of an alkene. E1 reactions are favored in conditions similar to those for SN1 reactions, such as the use of polar protic solvents and tertiary substrates.
E2 Reactions: Bimolecular Elimination
E2 reactions are bimolecular elimination reactions, where the rate-determining step involves the simultaneous removal of a leaving group and a beta-hydrogen by a strong base, resulting in the formation of an alkene. Unlike E1 reactions, E2 reactions do not involve the formation of a carbocation intermediate and are typically favored with primary substrates and strong bases in polar aprotic solvents. The stereochemistry of E2 reactions often results in the most stable alkene product, due to the concerted nature of the reaction mechanism.
Key Points
- SN1 reactions are unimolecular and involve the formation of a carbocation intermediate, typically occurring with tertiary substrates.
- SN2 reactions are bimolecular, involving a concerted mechanism with stereochemical inversion, typically occurring with primary substrates.
- E1 reactions are unimolecular eliminations that also involve the formation of a carbocation intermediate, similar to SN1 reactions but resulting in alkene formation.
- E2 reactions are bimolecular eliminations involving a concerted mechanism with a strong base, resulting in alkene formation and typically occurring with primary substrates.
- The choice of solvent and substrate can significantly influence the outcome of these reactions, with polar protic solvents favoring SN1 and E1 reactions, and polar aprotic solvents favoring SN2 and E2 reactions.
Understanding the mechanisms and conditions under which SN1, SN2, E1, and E2 reactions occur is essential for predicting and controlling the outcomes of organic reactions. These concepts form the foundation of organic chemistry and are critical for the synthesis of complex molecules and the explanation of reaction mechanisms. By recognizing the factors that influence the choice between these reaction pathways, chemists can design and optimize synthetic routes to desired products, leveraging the unique characteristics of each reaction type to achieve specific outcomes.
What is the primary difference between SN1 and SN2 reactions?
+The primary difference between SN1 and SN2 reactions lies in their mechanisms. SN1 reactions are unimolecular, involving the formation of a carbocation intermediate, while SN2 reactions are bimolecular, involving a concerted mechanism with stereochemical inversion.
Under what conditions do E1 and E2 reactions typically occur?
+E1 reactions typically occur under conditions similar to SN1 reactions, such as with tertiary substrates in polar protic solvents. E2 reactions, on the other hand, are favored with primary substrates, strong bases, and polar aprotic solvents.
How do the choice of solvent and substrate influence the outcome of these reactions?
+The choice of solvent and substrate can significantly influence the outcome of these reactions. Polar protic solvents favor SN1 and E1 reactions by stabilizing the carbocation intermediate, while polar aprotic solvents favor SN2 and E2 reactions by facilitating the approach of the nucleophile or base to the substrate.
In conclusion, SN1, SN2, E1, and E2 reactions represent fundamental concepts in organic chemistry, each with its unique mechanism and set of conditions under which it occurs. Mastering these concepts is essential for understanding and predicting the outcomes of organic reactions, allowing chemists to design efficient synthetic strategies and explain complex reaction mechanisms. By recognizing the interplay between substrate, solvent, and reaction conditions, chemists can harness the power of these reactions to achieve specific synthetic goals, contributing to advancements in fields ranging from pharmaceuticals to materials science.