The water phase diagram is a fundamental concept in physics and chemistry, describing the different states of water under various conditions of temperature and pressure. It's a crucial tool for understanding the behavior of water in different environments, from the Earth's atmosphere to industrial processes. In this article, we will explore 5 ways the water phase diagram works, delving into its intricacies and applications.
Key Points
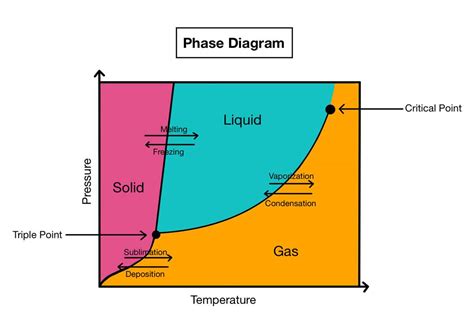
- The water phase diagram illustrates the relationship between temperature, pressure, and the states of water (solid, liquid, gas).
- The diagram is divided into three main regions: solid (ice), liquid (water), and gas (water vapor), with boundaries marking the phase transitions.
- Phase transitions occur at specific points on the diagram, such as the melting point (0°C at standard pressure) and the boiling point (100°C at standard pressure).
- The water phase diagram is essential in various fields, including meteorology, engineering, and biology, for predicting and understanding water's behavior under different conditions.
- Understanding the water phase diagram is crucial for managing water resources, predicting weather patterns, and developing technologies that rely on water, such as desalination plants and cooling systems.
Understanding the Basics of the Water Phase Diagram
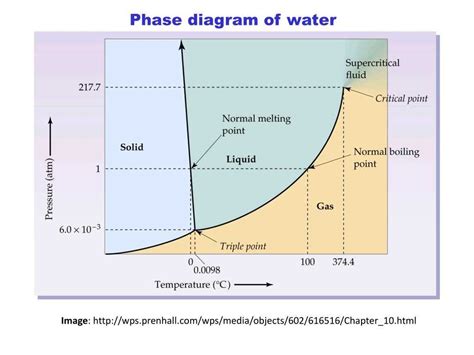
The water phase diagram is typically represented as a graph with temperature on the x-axis and pressure on the y-axis. The diagram shows the three main phases of water: solid (ice), liquid (water), and gas (water vapor). The boundaries between these phases mark the phase transitions, where water changes from one state to another. For example, the melting point of ice is 0°C at standard pressure (1013 mbar), and the boiling point of water is 100°C at the same pressure.
Phase Transitions and Critical Points
Phase transitions are critical aspects of the water phase diagram. They occur when the temperature and pressure conditions are such that water changes its state. The most common phase transitions are melting (solid to liquid), freezing (liquid to solid), vaporization (liquid to gas), and condensation (gas to liquid). The water phase diagram also includes a critical point, which is the temperature and pressure above which the distinction between the liquid and gas phases disappears. For water, this critical point is at approximately 374°C and 221 bar.
| Phase Transition | Temperature (°C) | Pressure (mbar) |
|---|---|---|
| Melting Point | 0 | 1013 |
| Boiling Point | 100 | 1013 |
| Critical Point | 374 | 221 bar (22100 mbar) |
Applications of the Water Phase Diagram
The water phase diagram has numerous applications across various fields. In meteorology, it’s used to understand and predict weather patterns, including the formation of clouds and precipitation. Engineers rely on the diagram to design systems that involve water, such as cooling systems, desalination plants, and steam turbines. In biology, the water phase diagram is essential for understanding the behavior of water in living organisms and the effects of temperature and pressure on biological processes.
Industrial and Environmental Applications
In industry, the water phase diagram is crucial for optimizing processes that involve water, such as power generation, chemical manufacturing, and food processing. It helps in designing more efficient systems, predicting potential issues, and ensuring the safety of operations. Environmentally, understanding the water phase diagram is vital for managing water resources, predicting the effects of climate change, and developing strategies for water conservation and sustainability.
As we delve deeper into the applications and implications of the water phase diagram, it becomes clear that its significance extends far beyond the realm of pure physics or chemistry. It's a foundational concept that underpins our understanding of the natural world and informs our approaches to technological innovation and environmental stewardship.
What is the significance of the water phase diagram in everyday life?
+The water phase diagram is crucial for understanding and predicting the behavior of water in various conditions, which is essential for managing water resources, predicting weather patterns, and designing technologies that rely on water.
How does the water phase diagram affect the formation of clouds and precipitation?
+The water phase diagram helps in understanding the conditions under which water vapor in the atmosphere condenses into clouds and precipitates as rain or snow. This knowledge is vital for meteorological predictions and understanding climate patterns.
What role does the water phase diagram play in the design of cooling systems and steam turbines?
+The water phase diagram is essential for designing efficient cooling systems and steam turbines. By understanding the phase transitions of water, engineers can optimize the performance of these systems, ensuring they operate safely and efficiently under various conditions.
In conclusion, the water phase diagram is a powerful tool that not only explains the behavior of water under different conditions but also has profound implications for various aspects of our lives, from the environment and industry to our daily experiences with weather and technology. Its significance underscores the importance of continued research and education in the sciences, as understanding such fundamental concepts is key to advancing our knowledge and addressing the challenges of the future.