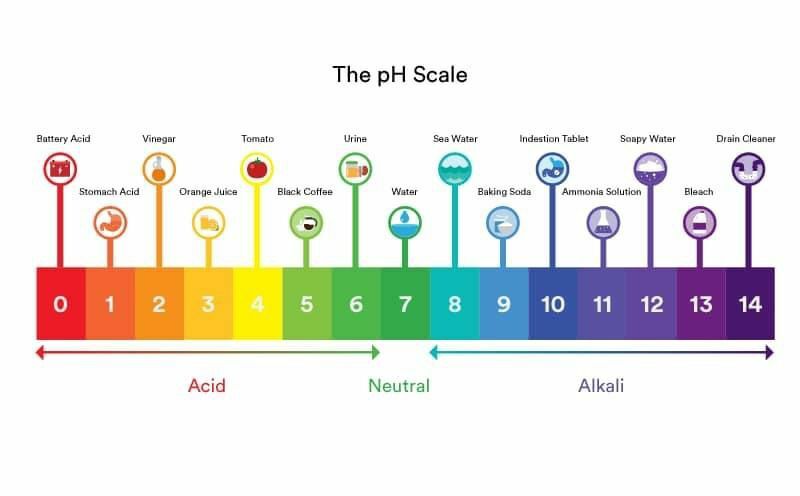
The pH of vinegar is a topic of interest in various fields, including culinary arts, chemistry, and food science. Vinegar, a diluted solution of acetic acid, has a distinctive pH level that contributes to its characteristic properties and uses. Understanding the pH of vinegar requires a brief exploration of its chemical composition and the pH scale.
The pH scale, ranging from 0 to 14, measures the concentration of hydrogen ions in a solution, with lower values indicating higher acidity and higher values indicating higher alkalinity. A pH of 7 is considered neutral, as it is the pH of pure water. The pH of vinegar typically falls in the acidic range, with most types of vinegar having a pH between 2.4 and 3.4. This acidity is due to the presence of acetic acid (CH3COOH), the primary component of vinegar, which dissociates into hydrogen ions (H+) and acetate ions (CH3COO-) in aqueous solution.
Key Points
- Vinegar's pH level, usually between 2.4 and 3.4, is due to its acetic acid content.
- The pH of vinegar can vary depending on the type, production method, and intended use.
- Understanding vinegar's pH is crucial for its applications in cooking, food preservation, and industrial processes.
- Acetic acid, the main component of vinegar, is responsible for its acidic properties and potential health benefits.
- The pH level of vinegar can affect its interaction with other substances and its suitability for various purposes.
Variations in Vinegar pH
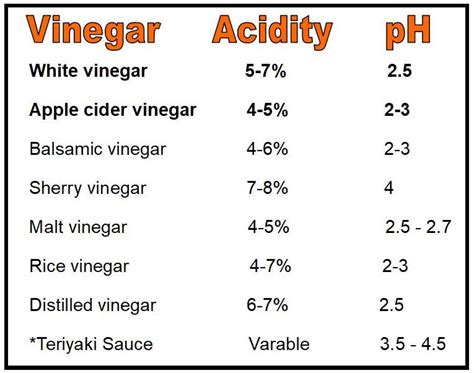
The pH of vinegar can vary slightly depending on the type of vinegar, its production method, and its intended use. For example, white vinegar, also known as distilled vinegar, tends to have a lower pH (around 2.4) due to its higher acetic acid concentration, typically around 5%. Apple cider vinegar, on the other hand, might have a slightly higher pH (around 3.0) because it can contain additional compounds that buffer the acidity. Balsamic vinegar, known for its rich flavor and thicker consistency, can have a pH ranging from 2.8 to 3.4, reflecting its complex composition and aging process.
Factors Influencing Vinegar pH
Several factors can influence the pH of vinegar, including the raw materials used for its production, the fermentation process, and any post-fermentation treatments. The natural presence of other acids, such as citric acid or malic acid, can also affect the overall pH. Additionally, the method of dilution and the presence of any additives can further influence the final pH of the vinegar product. Understanding these factors is essential for producing vinegar with specific properties for various applications, from culinary uses to industrial processes.
| Type of Vinegar | Typical pH Range | Acetic Acid Concentration |
|---|---|---|
| White Vinegar | 2.4 - 2.7 | 4-6% |
| Apple Cider Vinegar | 2.8 - 3.0 | 4-6% |
| Balsamic Vinegar | 2.8 - 3.4 | 6-8% |
| Wine Vinegar | 2.5 - 3.0 | 5-7% |
Applications of Vinegar and pH Considerations

The pH of vinegar plays a significant role in its various applications. In cooking, the acidity of vinegar can help balance flavors, preserve foods by inhibiting bacterial growth, and contribute to the texture of certain dishes. For instance, pickling vegetables often involves soaking them in a brine solution that includes vinegar, which helps create an acidic environment that preserves the food. The choice of vinegar for such applications can depend on the desired flavor profile and the pH level required for the specific food preservation or culinary technique.
Health and pH Considerations
There is also interest in the potential health benefits of vinegar, particularly apple cider vinegar, due to its acidity and the presence of other compounds like polyphenols. Some proponents suggest that consuming apple cider vinegar can help with digestion, weight management, and blood sugar control, possibly due to its pH level and the way it interacts with the body’s digestive system. However, it’s essential to approach such claims with a critical perspective, considering the need for further research and the importance of consulting healthcare professionals before using vinegar or any other substance for health purposes.
In conclusion, the pH of vinegar is a complex topic that reflects the chemical composition of vinegar and its various applications. Understanding the pH levels of different types of vinegar and the factors that influence these levels can provide insights into the selection and use of vinegar in culinary, industrial, and potential health contexts. As with any topic involving chemistry and health, a balanced and informed approach is crucial for maximizing the benefits of vinegar while minimizing potential risks.
What is the typical pH range of vinegar?
+The typical pH range of vinegar is between 2.4 and 3.4, depending on the type and production method.
How does the pH of vinegar affect its use in cooking?
+The pH of vinegar can affect its interaction with other ingredients, its ability to preserve foods, and the overall flavor profile of dishes. Choosing the right type of vinegar for a specific recipe can enhance the culinary experience.
Can the pH of vinegar influence its potential health benefits?
+While there is some evidence suggesting that vinegar, particularly apple cider vinegar, may have health benefits due to its acidity and other compounds, it’s essential to consult healthcare professionals and consider the need for further research before using vinegar for health purposes.