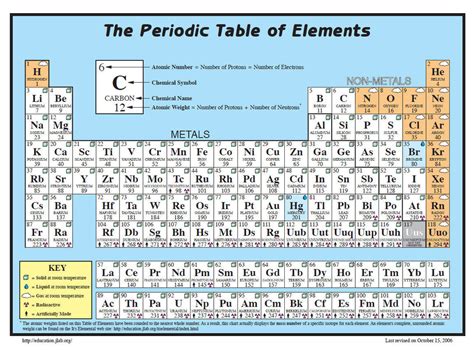
The Periodic Table is a fundamental tool in chemistry, providing a systematic organization of elements based on their atomic structure and properties. One of the critical pieces of information associated with each element is its molar mass, which is the mass of one mole of that element. Understanding and calculating molar masses is essential for various chemical calculations, including determining the amount of substance in a given sample and calculating the yield of chemical reactions. In this comprehensive guide, we will delve into the concept of molar mass, its significance in chemistry, and how to use the Periodic Table to find and calculate molar masses of elements.
Introduction to Molar Mass
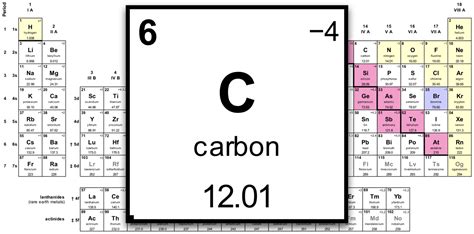
Molar mass is defined as the mass of one mole of a substance. It is expressed in units of grams per mole (g/mol) and represents the total mass of atoms in one mole of a substance. The molar mass of an element is determined by the sum of the masses of its naturally occurring isotopes, weighted by their abundance. This concept is crucial in chemistry because it allows for the conversion between the amount of substance (measured in moles) and the mass of a substance (measured in grams), facilitating a wide range of calculations in chemical reactions and analyses.
Key Points
- The molar mass of an element is the mass of one mole of that element, expressed in g/mol.
- It is calculated based on the atomic masses of the element's isotopes and their relative abundance.
- Molar mass is essential for calculating the amount of substance in a sample and predicting the outcomes of chemical reactions.
- The Periodic Table provides the atomic masses of elements, which can be used to calculate their molar masses.
- Understanding molar mass is fundamental for stoichiometry, the part of chemistry that deals with the relative quantities of reactants and products in chemical reactions.
Understanding the Periodic Table

The Periodic Table is arranged in a way that elements with similar properties recur at regular intervals. Elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. The atomic mass of each element, which is very close to its molar mass for monatomic substances, is typically listed below the element’s symbol on the Periodic Table. For elements that consist of molecules (such as oxygen, O2, and nitrogen, N2) in their standard state, the molar mass is the sum of the atomic masses of the atoms in one molecule of the substance.
Calculating Molar Mass
For elements that are monatomic (exist as single atoms) in their standard state, such as helium (He) and neon (Ne), the molar mass is essentially the atomic mass listed on the Periodic Table. For elements that exist as diatomic molecules (two atoms bonded together) in their standard state, like hydrogen (H2), oxygen (O2), nitrogen (N2), and fluorine (F2), the molar mass is twice the atomic mass of the element. For more complex molecules, the molar mass is the sum of the atomic masses of all atoms in the molecule, taking into account the multiplicity of each type of atom.
| Element | Atomic Mass (g/mol) | Standard State | Molar Mass (g/mol) |
|---|---|---|---|
| Helium (He) | 4.0026 | Monatomic | 4.0026 |
| Oxygen (O) | 15.9994 | Diatomic (O2) | 31.9988 |
| Nitrogen (N) | 14.0067 | Diatomic (N2) | 28.0134 |
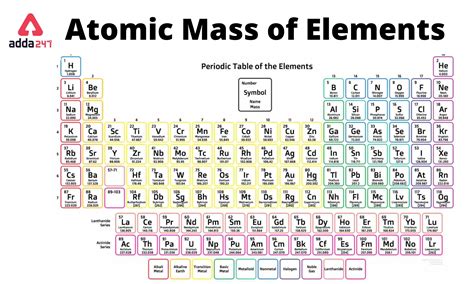
Practical Applications of Molar Mass
Molar mass has numerous practical applications in chemistry and related fields. It is used in the calculation of the empirical and molecular formulas of compounds, the determination of the percentage composition of elements in a compound, and the calculation of yields and reactant quantities in chemical reactions. Understanding molar mass is also essential in pharmaceutical applications, where the precise dosing of medications depends on the accurate calculation of molar quantities.
Chemical Reactions and Stoichiometry
In chemical reactions, molar mass is critical for determining the stoichiometry of the reaction, which describes the quantitative relationship between reactants and products. By knowing the molar masses of the reactants and products, chemists can calculate the amount of each substance needed for a reaction and predict the amount of product that will be formed, assuming the reaction goes to completion. This is fundamental for optimizing chemical processes, ensuring safety, and minimizing waste in industrial and laboratory settings.
What is the difference between atomic mass and molar mass?
+Atomic mass refers to the mass of a single atom of an element, while molar mass is the mass of one mole of that element. For monatomic elements, these values are essentially the same, but for elements that exist as molecules, the molar mass is the sum of the atomic masses of the atoms in one molecule.
How do you calculate the molar mass of a compound?
+The molar mass of a compound is calculated by summing the atomic masses of all atoms in the molecular formula of the compound, taking into account the multiplicity of each type of atom.
Why is molar mass important in chemistry?
+Molar mass is crucial for converting between the amount of substance (in moles) and the mass of a substance (in grams), facilitating various calculations in chemical reactions, stoichiometry, and pharmaceutical applications.
In conclusion, understanding molar mass and how to calculate it using the Periodic Table is fundamental for any study or application in chemistry. The ability to convert between moles and grams of a substance, calculate the composition of compounds, and predict the outcomes of chemical reactions relies heavily on the concept of molar mass. As chemistry continues to advance and play a critical role in addressing global challenges, the importance of molar mass in chemical education, research, and industry will only continue to grow.