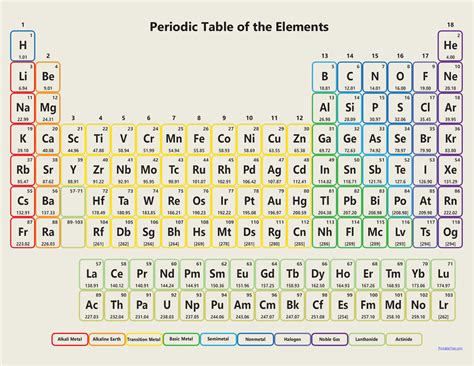
The Periodic Table of Elements is a fundamental tool in chemistry, providing a systematic and organized way to understand the properties and relationships of the elements. As of 2022, the Periodic Table consists of 118 confirmed elements, with the most recent additions being Nihonium (Nh), Moscovium (Mc), Tennessine (Ts), and Oganesson (Og) in 2016.
Introduction to the Periodic Table
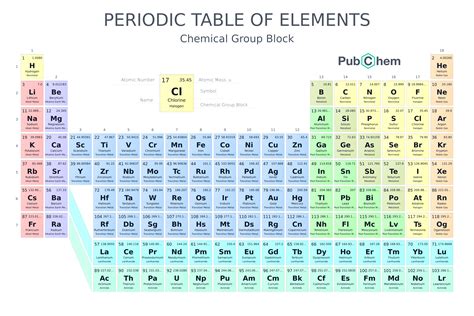
The Periodic Table is arranged in a logical and symmetrical manner, with elements listed in order of increasing atomic number (number of protons in the nucleus). The table is divided into rows called periods and columns called groups or families. Elements in the same group exhibit similar chemical properties due to the same number of electrons in their outermost shell.
Structure of the Periodic Table
The Periodic Table can be divided into several sections, including the s-block, p-block, d-block, and f-block. The s-block elements are found in the first two groups (alkali metals and alkaline earth metals) and are characterized by a single electron in their outermost s-orbital. The p-block elements are found in groups 13-18 and are characterized by electrons in their outermost p-orbitals. The d-block elements are found in the transition metal block (groups 3-12) and are characterized by electrons in their outermost d-orbitals. The f-block elements are found at the bottom of the table and are characterized by electrons in their outermost f-orbitals.
| Block | Groups | Elements |
|---|---|---|
| s-block | 1-2 | Alkali metals, alkaline earth metals |
| p-block | 13-18 | Nonmetals, metalloids, and post-transition metals |
| d-block | 3-12 | Transition metals |
| f-block | Lanthanides and actinides | Inner transition metals |
Properties of Elements
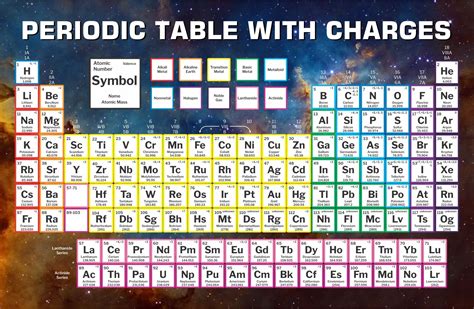
Elements in the same group exhibit similar chemical properties due to the same number of electrons in their outermost shell. These properties include atomic radius, electronegativity, and reactivity. Atomic radius increases down a group and decreases across a period, while electronegativity increases across a period and decreases down a group.
Chemical Properties
Chemical properties of elements are determined by the number of electrons in their outermost shell. Elements in the same group exhibit similar chemical properties, such as reactivity with other elements. The noble gases (group 18) are unreactive due to their full outermost shell, while the alkali metals (group 1) are highly reactive due to their single electron in their outermost s-orbital.
| Group | Chemical Properties |
|---|---|
| 1 (Alkali metals) | Highly reactive, single electron in outermost s-orbital |
| 2 (Alkaline earth metals) | Less reactive than alkali metals, two electrons in outermost s-orbital |
| 17 (Halogens) | Highly reactive, seven electrons in outermost p-orbital |
| 18 (Noble gases) | Unreactive, full outermost shell |
Key Points
- The Periodic Table is a systematic and organized way to understand the properties and relationships of the elements.
- Elements in the same group exhibit similar chemical properties due to the same number of electrons in their outermost shell.
- The Periodic Table is divided into several sections, including the s-block, p-block, d-block, and f-block.
- Chemical properties of elements are determined by the number of electrons in their outermost shell.
- Understanding the structure and organization of the Periodic Table is essential for chemists and researchers to identify patterns and relationships between elements.
Applications of the Periodic Table
The Periodic Table has numerous applications in chemistry, physics, and engineering. It is used to predict the properties of elements, identify patterns and relationships between elements, and understand the behavior of elements in different situations.
Chemical Research and Development
The Periodic Table is essential for chemical research and development, as it provides a framework for understanding the properties and relationships of elements. Chemists use the Periodic Table to predict the properties of new elements, design new compounds, and understand the behavior of elements in different situations.
What is the Periodic Table of Elements?
+The Periodic Table of Elements is a systematic and organized way to understand the properties and relationships of the elements.
How is the Periodic Table organized?
+The Periodic Table is organized in a logical and symmetrical manner, with elements listed in order of increasing atomic number (number of protons in the nucleus).
What are the applications of the Periodic Table?
+The Periodic Table has numerous applications in chemistry, physics, and engineering, including predicting the properties of elements, identifying patterns and relationships between elements, and understanding the behavior of elements in different situations.
In conclusion, the Periodic Table of Elements is a fundamental tool in chemistry, providing a systematic and organized way to understand the properties and relationships of the elements. Understanding the structure and organization of the Periodic Table is essential for chemists and researchers to identify patterns and relationships between elements, and to predict the properties of new elements. The Periodic Table has numerous applications in chemistry, physics, and engineering, and is an essential resource for anyone interested in the properties and behavior of the elements.