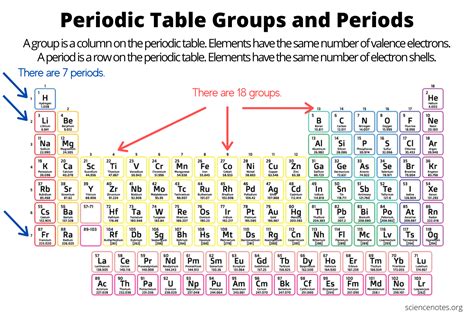
The periodic table is a fundamental tool in chemistry, used to organize elements based on their atomic number, electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. There are currently 7 period groups in the periodic table, each representing a row of elements. Understanding these period groups is crucial for comprehending the periodic trends and the chemical behavior of elements.
Introduction to Period Groups
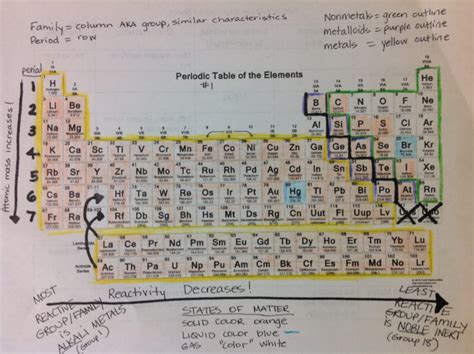
The periodic table’s structure is based on the periodic law, which states that the properties of elements recur periodically when the elements are arranged in order of increasing atomic number. The period groups are horizontal rows of elements, and the elements in each group exhibit similar chemical properties due to the same number of electrons in their outermost shell. The first period contains only two elements: hydrogen and helium. The subsequent periods contain more elements, with the number of elements in each period increasing due to the availability of more orbitals for electron filling.
First Period Group
The first period group consists of hydrogen (H) and helium (He). Hydrogen is the lightest and most abundant chemical element in the universe, and it has a unique position in the periodic table due to its ability to form compounds with almost all other elements. Helium, on the other hand, is a noble gas and does not readily react with other elements. The first period is the shortest period in the periodic table, reflecting the limited number of electrons that can occupy the 1s orbital.
| Element | Atomic Number | Electronic Configuration |
|---|---|---|
| Hydrogen (H) | 1 | 1s1 |
| Helium (He) | 2 | 1s2 |
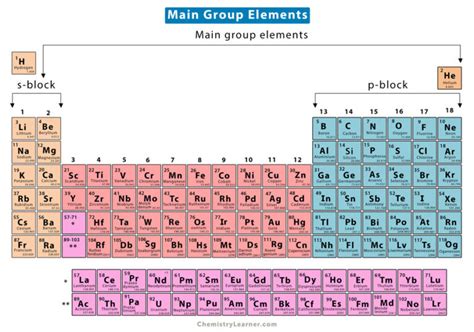
Second to Seventh Period Groups

The second period starts with lithium (Li) and ends with neon (Ne), introducing the s-block and p-block elements. The third period includes sodium (Na) to argon (Ar), with the addition of more electrons leading to a greater variety of chemical properties. The fourth period, from potassium (K) to krypton (Kr), sees the introduction of the d-block elements, which are characterized by the filling of the d orbitals. The fifth period, from rubidium (Rb) to xenon (Xe), includes the first f-block elements (lanthanides), which exhibit unique properties due to the filling of the f orbitals. The sixth period, from cesium (Cs) to radon (Rn), includes the actinides, a series of radioactive, metallic elements. The seventh period, starting with francium (Fr) and currently ending with oganesson (Og), is still being explored, with many of its elements synthesized in laboratories and having very short half-lives.
Chemical Properties and Trends
The chemical properties of elements in each period group are influenced by their electron configuration, particularly the number of electrons in the outermost shell. As you move from left to right across a period, the atomic radius decreases due to the increase in the effective nuclear charge (the net positive charge experienced by an electron in the outer shell), leading to elements becoming less metallic and more nonmetallic. The reactivity of elements also changes, with elements on the left side of the periodic table (alkali metals) being highly reactive and those on the right (noble gases) being unreactive.
Key Points
- The periodic table is structured into periods (rows) and groups (columns), with elements in the same group exhibiting similar chemical properties.
- Each period group represents a unique set of chemical properties and trends due to the electron configuration of the elements.
- The first period is the shortest, containing hydrogen and helium, while subsequent periods contain more elements due to the availability of more orbitals for electron filling.
- Understanding the period groups is essential for predicting the chemical behavior of elements and their potential applications.
- The chemical properties of elements in a period are influenced by the number of electrons in the outermost shell and the effective nuclear charge.
Practical Applications and Trends
The understanding of period groups and their chemical properties has numerous practical applications in chemistry, physics, and engineering. For instance, the reactivity series, which is based on the reactivity of metals, is crucial for predicting the outcomes of chemical reactions and for the design of electrochemical cells. The periodic trends also play a significant role in materials science, where the properties of elements are used to develop new materials with specific characteristics, such as superconductors, nanomaterials, and catalysts.
Future Developments and Implications
As research continues to uncover new elements and understand their properties, the periodic table remains a dynamic tool. The synthesis of new elements in the seventh period and beyond will continue to challenge our understanding of periodic trends and the properties of matter at the atomic level. Furthermore, advancements in materials science and technology will increasingly rely on the precise manipulation of elemental properties, making a deep understanding of the periodic table and its period groups essential for future innovations.
What is the significance of understanding the period groups in the periodic table?
+Understanding the period groups is crucial for predicting the chemical behavior of elements, including their reactivity, electron configuration, and potential applications in various fields such as materials science and chemistry.
How do the chemical properties of elements change across a period?
+As you move from left to right across a period, the atomic radius decreases, and the elements become less metallic and more nonmetallic. The reactivity also changes, with elements on the left being highly reactive and those on the right being less reactive.
What are some practical applications of understanding the periodic trends and period groups?
+The understanding of periodic trends and period groups has applications in materials science, chemistry, and physics, including the development of new materials, prediction of chemical reactions, and design of electrochemical cells.
In conclusion, the 7 period groups of the periodic table offer a structured approach to understanding the chemical properties and trends of elements. By recognizing the patterns and relationships within these groups, scientists can predict the behavior of elements, develop new materials, and advance our understanding of the atomic world. As the periodic table continues to evolve with the discovery of new elements, its role as a fundamental tool in chemistry and beyond remains unwavering.