The phosphorus trichloride (PCL3) molecule is a key subject of study in chemistry, particularly when it comes to understanding its electron geometry. Electron geometry refers to the arrangement of electron groups around a central atom, which in this case is phosphorus. The electron geometry of PCL3 is determined by the number of electron groups around the phosphorus atom and the number of bonding and lone pairs. Here, we will explore 5 key aspects of PCL3 electron geometry, providing insights into its structure and properties.
Understanding Electron Groups and Geometry
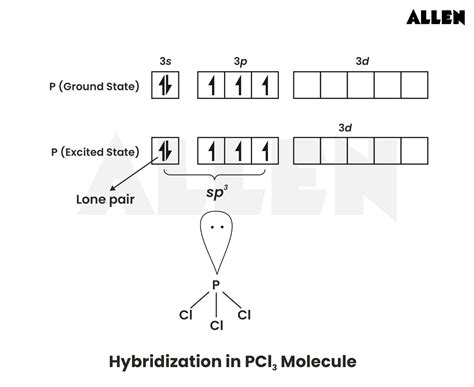
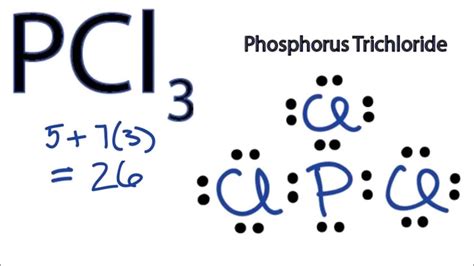
In the context of VSEPR (Valence Shell Electron Pair Repulsion) theory, electron groups are defined as regions around a central atom where electrons are likely to be found. For PCL3, there are three bonding pairs (each representing a covalent bond with a chlorine atom) and one lone pair on the phosphorus atom. The total number of electron groups around the phosphorus atom is four, which influences the molecule’s electron geometry. The electron geometry of a molecule with four electron groups is tetrahedral, but the actual molecular shape can differ due to the presence of lone pairs.
Determining Molecular Shape
The molecular shape of PCL3 is determined by the arrangement of its atoms in space. With three bonding pairs and one lone pair, PCL3 adopts a trigonal pyramidal shape. This is because the lone pair occupies more space than the bonding pairs due to greater electron-electron repulsion, causing the bonding pairs (and thus the chlorine atoms) to be pushed closer together. The result is a molecule with a triangular base (where the chlorine atoms are located) and the phosphorus atom at the apex, with the lone pair taking up the space opposite the base.
| Electron Group | Description |
|---|---|
| Bonding Pairs | 3 (each representing a P-Cl bond) |
| Lone Pairs | 1 (on the phosphorus atom) |
| Total Electron Groups | 4 |
| Electron Geometry | Tetrahedral |
| Molecular Shape | Trigonal Pyramidal |
Implications of Electron Geometry on Physical Properties
The electron geometry and molecular shape of PCL3 have significant implications for its physical properties. For instance, the polarity of the molecule is influenced by its shape. The trigonal pyramidal shape of PCL3 results in a net dipole moment, making it a polar molecule. This polarity affects the molecule’s boiling point, solubility, and reactivity. Furthermore, the electron geometry influences the molecule’s stability and its ability to participate in chemical reactions, particularly those involving the lone pair on the phosphorus atom.
Chemical Reactivity and Electron Geometry
The chemical reactivity of PCL3 is also closely related to its electron geometry. The lone pair on the phosphorus atom makes it a good nucleophile, capable of donating a pair of electrons to form new bonds. This property is essential in many chemical reactions, including substitution reactions where PCL3 acts as a reactant. Understanding the electron geometry and the role of the lone pair is crucial for predicting and explaining the outcomes of such reactions.
Key Points
- The electron geometry of PCL3 is tetrahedral due to the presence of four electron groups around the phosphorus atom.
- The molecular shape of PCL3 is trigonal pyramidal, resulting from the arrangement of three bonding pairs and one lone pair.
- The polarity of PCL3, influenced by its trigonal pyramidal shape, affects its physical properties such as boiling point and solubility.
- The electron geometry and molecular shape play a significant role in determining the chemical reactivity of PCL3, particularly in reactions involving the lone pair on the phosphorus atom.
- Understanding the distinction between electron geometry and molecular shape is essential for predicting the properties and behaviors of molecules like PCL3.
In conclusion, the electron geometry of PCL3, determined by the arrangement of its electron groups, is a fundamental aspect of its chemical and physical properties. The tetrahedral electron geometry and trigonal pyramidal molecular shape of PCL3 have significant implications for its polarity, reactivity, and participation in chemical reactions. By understanding these concepts, chemists can better predict and explain the behavior of PCL3 and similar molecules, contributing to advancements in chemistry and related fields.
What is the electron geometry of PCL3?
+The electron geometry of PCL3 is tetrahedral, as determined by the four electron groups (three bonding pairs and one lone pair) around the phosphorus atom.
Why does PCL3 have a trigonal pyramidal molecular shape?
+PCL3 has a trigonal pyramidal molecular shape because the lone pair on the phosphorus atom occupies more space than the bonding pairs, causing the chlorine atoms to be pushed closer together and resulting in a triangular base with the phosphorus atom at the apex.
How does the electron geometry of PCL3 influence its chemical reactivity?
+The electron geometry of PCL3, particularly the presence of a lone pair on the phosphorus atom, makes it a good nucleophile. This property is essential for its participation in various chemical reactions, including substitution reactions.



