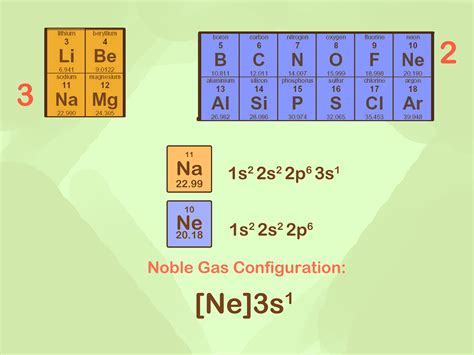
The noble gas configuration is a fundamental concept in chemistry, particularly in the realm of atomic structure and electron configuration. It refers to the arrangement of electrons in an atom that is similar to that of a noble gas, which is a group of elements in the periodic table known for their unreactivity and stability. The noble gases are located in group 18 of the periodic table and include elements such as helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
To understand the noble gas configuration, it is essential to delve into the basics of electron configuration. Electron configuration is the arrangement of electrons in an atom, which is determined by the number of protons in the nucleus and the energy levels that the electrons occupy. The electrons in an atom are arranged in different energy levels or shells, and each shell can hold a specific number of electrons. The first energy level, also known as the 1s orbital, can hold up to 2 electrons, while the second energy level, which includes the 2s and 2p orbitals, can hold up to 8 electrons.
The noble gas configuration is achieved when an atom has a full outer energy level, similar to that of a noble gas. This means that the outermost energy level is completely filled with electrons, resulting in a stable configuration. For example, the electron configuration of neon is 1s² 2s² 2p⁶, which indicates that the outermost energy level is fully occupied by 8 electrons. This full outer energy level is what gives noble gases their stability and unreactivity, as they do not readily form bonds with other atoms.
The noble gas configuration is significant in chemistry because it helps to explain the reactivity and stability of elements. Elements that have a noble gas configuration are generally unreactive and stable, while those that do not have a full outer energy level are more reactive. This concept is also crucial in understanding the formation of chemical bonds and the properties of different compounds.
Key Points
- The noble gas configuration refers to the arrangement of electrons in an atom that is similar to that of a noble gas.
- Elements with a noble gas configuration have a full outer energy level, resulting in stability and unreactivity.
- The noble gas configuration is significant in chemistry because it helps to explain the reactivity and stability of elements.
- Understanding the noble gas configuration is essential for understanding the formation of chemical bonds and the properties of different compounds.
- The noble gas configuration is achieved when an atom has a full outer energy level, similar to that of a noble gas.
Understanding Electron Configuration
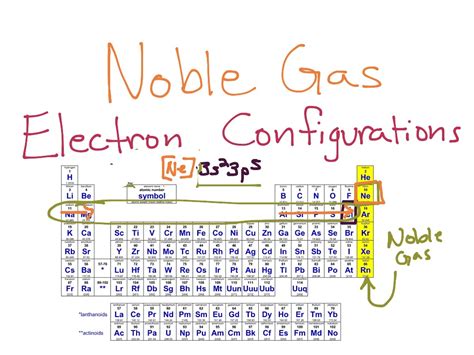
Electron configuration is the arrangement of electrons in an atom, which is determined by the number of protons in the nucleus and the energy levels that the electrons occupy. The electrons in an atom are arranged in different energy levels or shells, and each shell can hold a specific number of electrons. The first energy level, also known as the 1s orbital, can hold up to 2 electrons, while the second energy level, which includes the 2s and 2p orbitals, can hold up to 8 electrons.
Energy Levels and Orbitals
The energy levels in an atom are further divided into orbitals, which are regions around the nucleus where electrons are likely to be found. The orbitals are designated by letters such as s, p, d, and f, and each orbital has a specific shape and orientation in space. The s-orbitals are spherical in shape, while the p-orbitals are dumbbell-shaped. The d-orbitals are four-leaf clover-shaped, and the f-orbitals are six-leaf clover-shaped.
| Energy Level | Orbitals | Number of Electrons |
|---|---|---|
| 1st energy level | 1s | 2 |
| 2nd energy level | 2s, 2p | 8 |
| 3rd energy level | 3s, 3p, 3d | 18 |
| 4th energy level | 4s, 4p, 4d, 4f | 32 |

Applying the Noble Gas Configuration
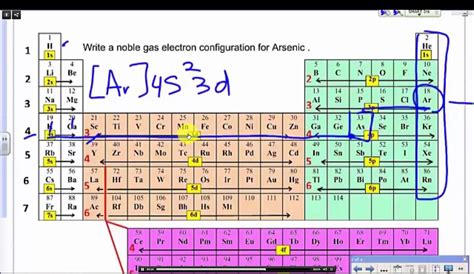
The noble gas configuration is applied in various areas of chemistry, including the prediction of chemical reactivity and the formation of chemical bonds. By understanding the electron configuration of an element, chemists can predict its reactivity and stability. For example, elements that have a noble gas configuration are generally unreactive and stable, while those that do not have a full outer energy level are more reactive.
Chemical Bonding
The noble gas configuration is also significant in understanding the formation of chemical bonds. Chemical bonds are formed when atoms share or exchange electrons to achieve a stable configuration. The noble gas configuration is a key factor in determining the type of bond that forms between atoms. For example, atoms that have a full outer energy level tend to form ionic bonds, while those that do not have a full outer energy level tend to form covalent bonds.
What is the noble gas configuration?
+The noble gas configuration refers to the arrangement of electrons in an atom that is similar to that of a noble gas.
Why is the noble gas configuration significant in chemistry?
+The noble gas configuration is significant in chemistry because it helps to explain the reactivity and stability of elements.
How is the noble gas configuration applied in chemistry?
+The noble gas configuration is applied in various areas of chemistry, including the prediction of chemical reactivity and the formation of chemical bonds.
Meta Description: Learn about the noble gas configuration and its significance in chemistry. Understand how the noble gas configuration is applied in predicting chemical reactivity and forming chemical bonds.