The concept of a Lewis structure is fundamental in chemistry, particularly in understanding the covalent bonding between atoms in molecules. Developed by Gilbert N. Lewis, these structures represent the arrangement of electrons in a molecule, using dots to symbolize electrons and lines to represent covalent bonds. However, there are instances where drawing a Lewis structure, as per the conventional rules, becomes impractical or impossible. This occurs when the molecule cannot satisfy the octet rule for all atoms, often due to an insufficient number of valence electrons or the presence of atoms that typically do not follow the octet rule, such as boron or atoms in the third period and beyond that can expand their octet.
Key Points
- Lewis structures are essential for visualizing molecular bonding and electron distribution.
- Certain molecules, due to their electron deficiency or excess, cannot be accurately represented by a traditional Lewis structure.
- Alternatives and extensions to Lewis structures, such as resonance structures and expanded octets, are used to address these limitations.
- The inability to draw a Lewis structure for some molecules highlights the complexity and variability of chemical bonding.
- Understanding these limitations is crucial for advancing chemical knowledge and developing new theories and models.
Limitations of Lewis Structures
Lewis structures are based on a set of rules designed to maximize the number of electrons in the valence shell of each atom, typically aiming for a full outer shell (octet) for non-hydrogen atoms. However, these rules are not universally applicable. Molecules with an odd number of electrons, or those with atoms that naturally do not fulfill the octet rule (like boron in BH3), pose significant challenges. Furthermore, molecules that exhibit delocalization of electrons across the molecule cannot be adequately represented by a single Lewis structure, necessitating the use of resonance structures to convey the distribution of electrons.
Odd-Electron Molecules
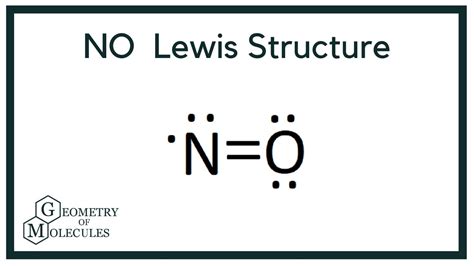
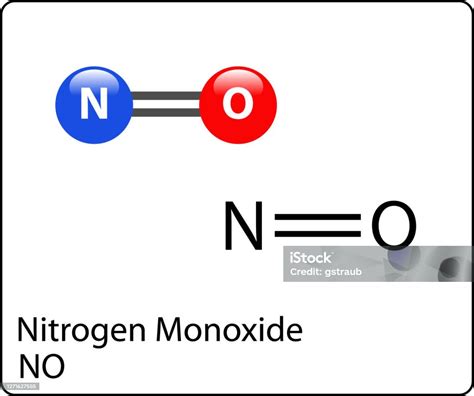
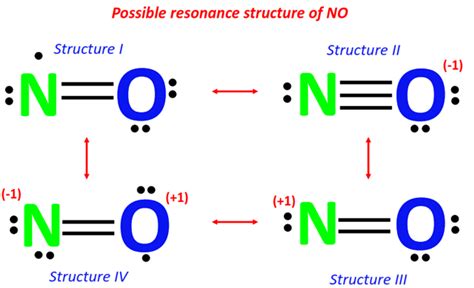
Molecules with an odd number of electrons are a prime example where a Lewis structure, in the traditional sense, cannot exist. These molecules, known as free radicals, have an unpaired electron that cannot be accommodated within the standard framework of Lewis structures. For instance, the nitric oxide molecule (NO) has 15 electrons (5 from nitrogen and 10 from oxygen), resulting in one unpaired electron. This unpaired electron cannot be paired in a Lewis structure without violating the basic principles of the model.
| Molecule | Number of Electrons | Challenge in Drawing Lewis Structure |
|---|---|---|
| Nitric Oxide (NO) | 15 | Odd number of electrons resulting in an unpaired electron |
| Boron Trifluoride (BF3) | 24 | Boron does not fulfill the octet rule in its ground state |
| Ozone (O3) | 18 | Delocalization of electrons requires resonance structures |
Alternatives and Extensions
To address the limitations of traditional Lewis structures, chemists employ several strategies, including resonance structures for molecules with delocalized electrons and expanded octets for atoms that can accommodate more than eight electrons in their valence shell. These extensions allow for a more comprehensive understanding of molecular structure and electron distribution, even if a single, definitive Lewis structure cannot be drawn.
Resonance Structures
For molecules where electrons are delocalized over the molecule, resonance structures are used. These structures represent different possible arrangements of electrons, none of which individually represent the molecule’s true structure but together provide a more accurate description. Ozone (O3) is a classic example, where two resonance structures are drawn to illustrate the delocalization of electrons across the oxygen atoms.
The use of resonance structures and other theoretical models highlights the evolving nature of chemical theory and the ongoing quest to better understand and describe the behavior of molecules. The recognition that no single model or structure can fully capture the complexity of molecular bonding and electron distribution underscores the dynamic and adaptive nature of chemical science.
What are the primary limitations of Lewis structures in chemistry?
+The primary limitations include the inability to accurately represent molecules with an odd number of electrons, those with atoms that naturally do not fulfill the octet rule, and molecules exhibiting significant electron delocalization.
How do chemists address these limitations?
+Chemists use alternative models such as resonance structures for delocalized electrons and consider expanded octets for atoms that can accommodate more than eight electrons, providing a more comprehensive understanding of molecular structure and reactivity.
What do the limitations of Lewis structures indicate about the nature of chemical bonding?
+The limitations highlight the complexity and variability of chemical bonding, indicating that molecular structure and electron distribution cannot always be simplified into a single, static representation. This complexity necessitates the use of multiple theoretical approaches and models to fully understand molecular behavior.
In conclusion, while Lewis structures are a foundational tool in chemistry for understanding molecular structure, their limitations are significant and reflect the inherent complexity of chemical bonding. The development of alternative models and the recognition of these limitations underscore the ongoing evolution of chemical theory and the pursuit of a deeper understanding of molecular behavior.