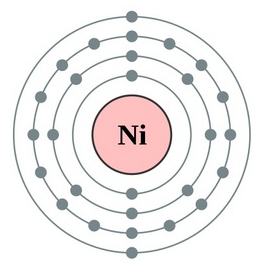
Nickel, a chemical element with the atomic number 28, is a versatile and widely used metal in various industries. Its unique properties make it an essential component in numerous applications, from coins and electronics to catalysts and alloys. Here are five key facts about nickel, focusing on its atomic number and related aspects:
Key Points
- Nickel's atomic number is 28, indicating it has 28 protons in its atomic nucleus.
- The atomic mass of nickel is approximately 58.6934 u (unified atomic mass units), which is a weighted average of its naturally occurring isotopes.
- Nickel is a transition metal, situated in the d-block of the periodic table, and its electron configuration is [Ar] 3d8 4s2.
- Nickel has a wide range of applications, including in stainless steel production, where it is alloyed with chromium and other metals to enhance corrosion resistance and strength.
- The discovery of nickel is attributed to Axel Cronstedt in 1751, although it was initially mistaken for a copper ore due to its appearance and properties.
Atomic Structure and Properties

Nickel’s atomic structure, with its 28 electrons arranged in a specific configuration, contributes to its chemical properties and reactivity. The electron configuration of nickel is [Ar] 3d8 4s2, which means it has a full outer energy level, making it relatively stable. However, nickel can exhibit multiple oxidation states, ranging from +1 to +4, due to the ease with which its electrons can be excited or removed.
Chemical Reactivity and Compounds
Nickel’s chemical reactivity is characterized by its ability to form compounds with a variety of elements, including oxygen, sulfur, and carbon. Nickel oxide (NiO), nickel sulfate (NiSO4), and nickel carbonyl (Ni(CO)4) are examples of such compounds. The formation of these compounds is influenced by nickel’s position in the periodic table and its electron configuration, which dictates its valency and the types of chemical bonds it can form.
| Property | Value |
|---|---|
| Atomic Number | 28 |
| Atomic Mass | 58.6934 u |
| Electron Configuration | [Ar] 3d8 4s2 |
| Oxidation States | +1, +2, +3, +4 |
Applications and Industrial Uses

Nickel’s applications are diverse, reflecting its versatile properties. In the production of stainless steel, nickel is alloyed with chromium and other metals to enhance corrosion resistance and strength. Nickel is also used in the manufacture of coins, due to its durability and resistance to corrosion. Furthermore, nickel’s catalytic properties make it useful in the petroleum industry for hydrogenation reactions, and its alloys are used in gas turbines and other high-temperature applications.
Environmental and Health Considerations
The use of nickel, like many metals, raises environmental and health concerns. Nickel can be toxic in certain forms, and exposure to nickel compounds has been linked to health issues, including dermatitis and respiratory problems. Additionally, the mining and processing of nickel can have significant environmental impacts, including pollution and habitat destruction. Therefore, the extraction, use, and disposal of nickel must be managed responsibly to minimize its adverse effects.
What is the primary use of nickel in industry?
+Nickel is primarily used in the production of stainless steel, where it is alloyed with chromium and other metals to enhance corrosion resistance and strength.
What are the common oxidation states of nickel?
+Nickel can exhibit multiple oxidation states, ranging from +1 to +4, due to the ease with which its electrons can be excited or removed.
Why is nickel used in coin production?
+Nickel is used in the manufacture of coins due to its durability and resistance to corrosion, making it a suitable material for circulating currencies.
In conclusion, nickel, with its atomic number of 28, is a chemically versatile element that plays a critical role in various industrial, technological, and everyday applications. Its unique properties, derived from its atomic structure and electron configuration, make it an indispensable component in the production of stainless steel, coins, catalysts, and alloys. As with any metal, the use of nickel must be balanced with considerations of environmental sustainability and human health to ensure its benefits are realized without undue cost.