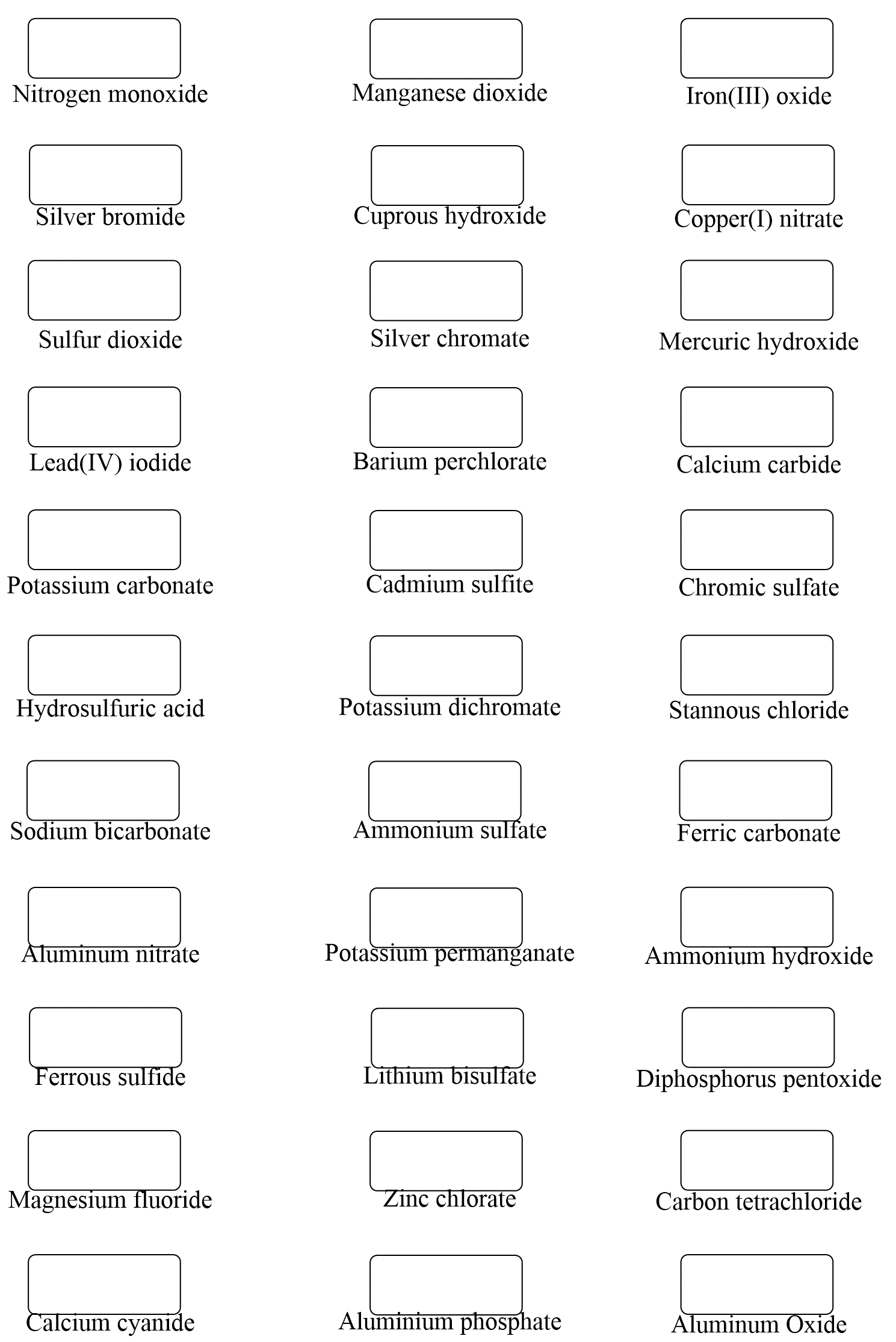
Naming compounds is a fundamental skill in chemistry, essential for effective communication among chemists and scientists. The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming compounds, which can seem complex at first, but with practice and understanding, become straightforward. In this article, we will delve into the world of naming compounds, exploring the basics, and providing a comprehensive guide to mastering this crucial aspect of chemistry.
Understanding the Basics of Compound Nomenclature

The IUPAC nomenclature system is based on a set of rules that provide a unique name for each compound. The system is designed to be logical and consistent, allowing chemists to easily identify and communicate the structure of a compound. The name of a compound typically consists of a prefix, infix, and suffix, which provide information about the compound’s structure and functional groups. For example, the compound methane has a prefix “meth-” indicating one carbon atom, and a suffix “-ane” indicating a saturated hydrocarbon.
Key Points
- IUPAC nomenclature provides a unique name for each compound
- The name consists of a prefix, infix, and suffix
- The prefix indicates the number of carbon atoms
- The suffix indicates the functional group or type of compound
- Understanding the basics of IUPAC nomenclature is essential for effective communication in chemistry
Naming Alkanes and Alkyl Groups
Alkanes are saturated hydrocarbons, and their names are derived from the number of carbon atoms in the molecule. The prefix for alkanes is based on the number of carbon atoms, with “meth-” indicating one carbon, “eth-” indicating two carbons, and so on. Alkyl groups are derived from alkanes by removing a hydrogen atom, and their names are formed by adding the suffix “-yl” to the prefix. For example, the alkyl group derived from methane is called methyl, and the alkyl group derived from ethane is called ethyl.
| Alkane | Prefix | Number of Carbon Atoms |
|---|---|---|
| Methane | Meth- | 1 |
| Ethane | Eth- | 2 |
| Propane | Prop- | 3 |
| Butane | But- | 4 |
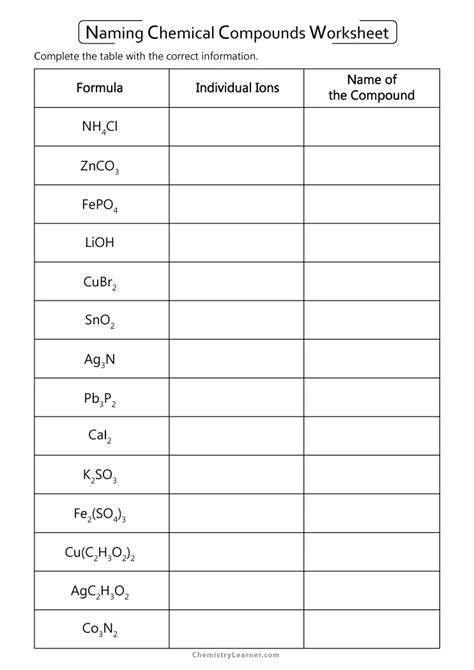
Naming Alkenes and Alkynes
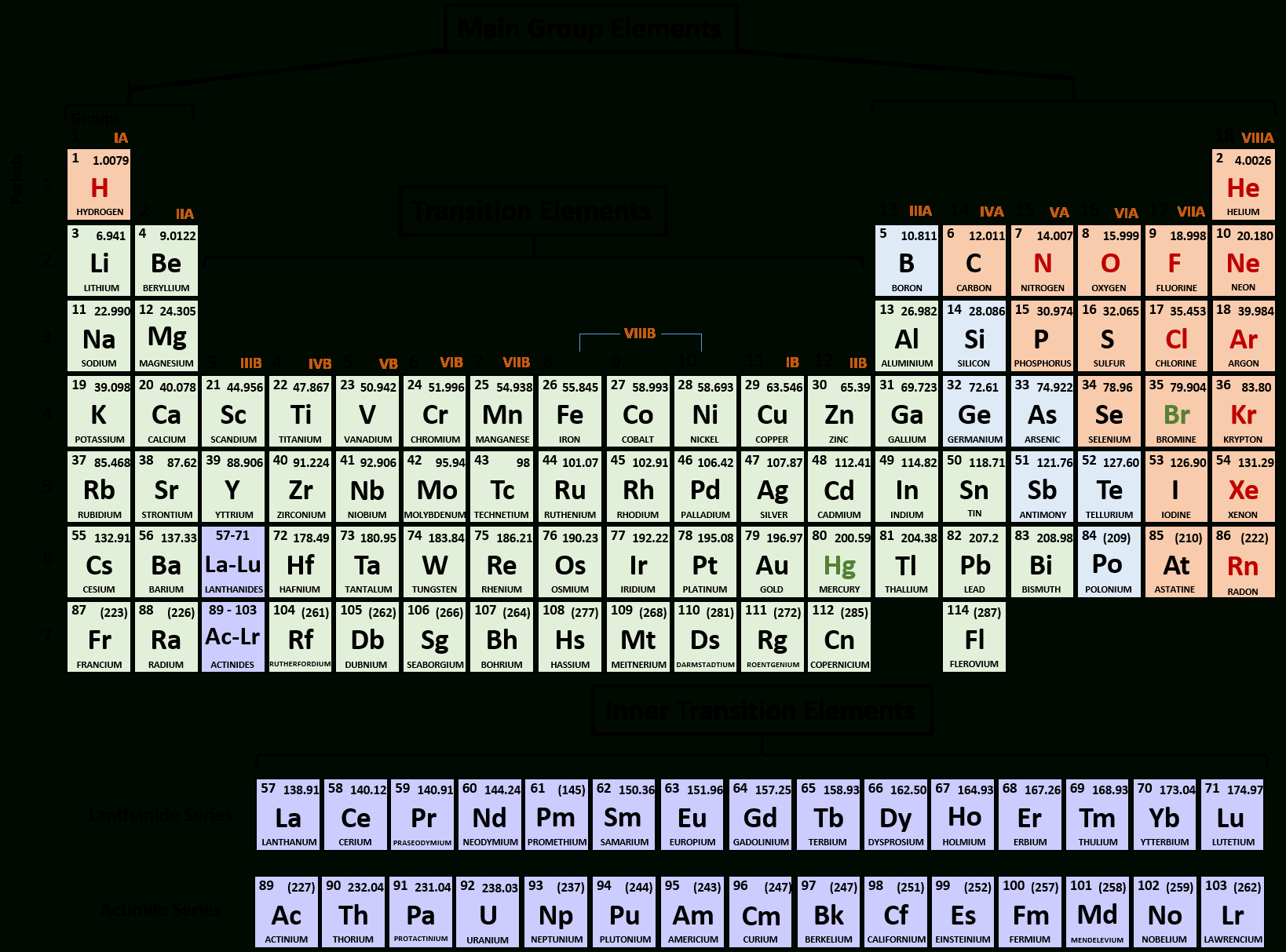
Alkenes are unsaturated hydrocarbons with one or more double bonds, and their names are derived from the corresponding alkane by replacing the suffix “-ane” with “-ene”. Alkynes are unsaturated hydrocarbons with one or more triple bonds, and their names are derived from the corresponding alkane by replacing the suffix “-ane” with “-yne”. For example, the compound ethene has one double bond, and the compound ethyne has one triple bond.
Naming Aromatic Compounds
Aromatic compounds are a class of compounds that contain a planar, ring-shaped molecule with alternating double bonds. The name of an aromatic compound is typically derived from the name of the corresponding alkane, with the suffix “-ane” replaced by “-ene” or “-yne”. For example, the compound benzene is an aromatic compound with a six-membered ring, and the compound toluene is an aromatic compound with a methyl group attached to the benzene ring.
What is the difference between an alkane and an alkene?
+An alkane is a saturated hydrocarbon, while an alkene is an unsaturated hydrocarbon with one or more double bonds.
How do you name an aromatic compound?
+The name of an aromatic compound is typically derived from the name of the corresponding alkane, with the suffix "-ane" replaced by "-ene" or "-yne".
What is the prefix for a compound with four carbon atoms?
+The prefix for a compound with four carbon atoms is "but-".
In conclusion, naming compounds is a crucial aspect of chemistry, and mastering the IUPAC nomenclature system is essential for effective communication among chemists and scientists. By understanding the basics of compound nomenclature, including the prefix, infix, and suffix, and practicing the naming of different types of compounds, such as alkanes, alkenes, alkynes, and aromatic compounds, chemists can develop a strong foundation in this fundamental skill.