The realm of chemistry is replete with intricacies, and one of the fundamental distinctions that underpin the study of chemical compounds is the difference between molecular and empirical formulas. These two types of formulas serve distinct purposes in understanding the composition and structure of chemical substances. In this comprehensive exploration, we will delve into the definitions, significance, and applications of both molecular and empirical formulas, highlighting their unique roles in the field of chemistry.
Key Points
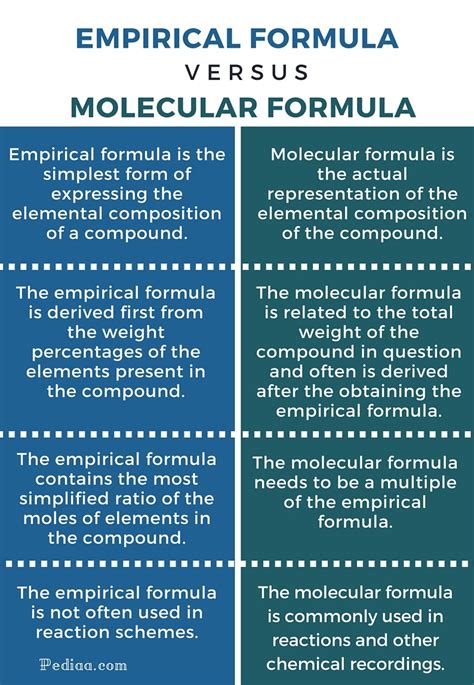
- Molecular formulas provide the exact number of atoms of each element in a molecule.
- Empirical formulas offer the simplest whole-number ratio of atoms of each element in a compound.
- The molecular formula is always a multiple of the empirical formula.
- Determining the molecular formula requires knowledge of the molecular weight or other structural information.
- Empirical formulas are useful for comparing compounds and determining their chemical properties.
Understanding Molecular Formulas
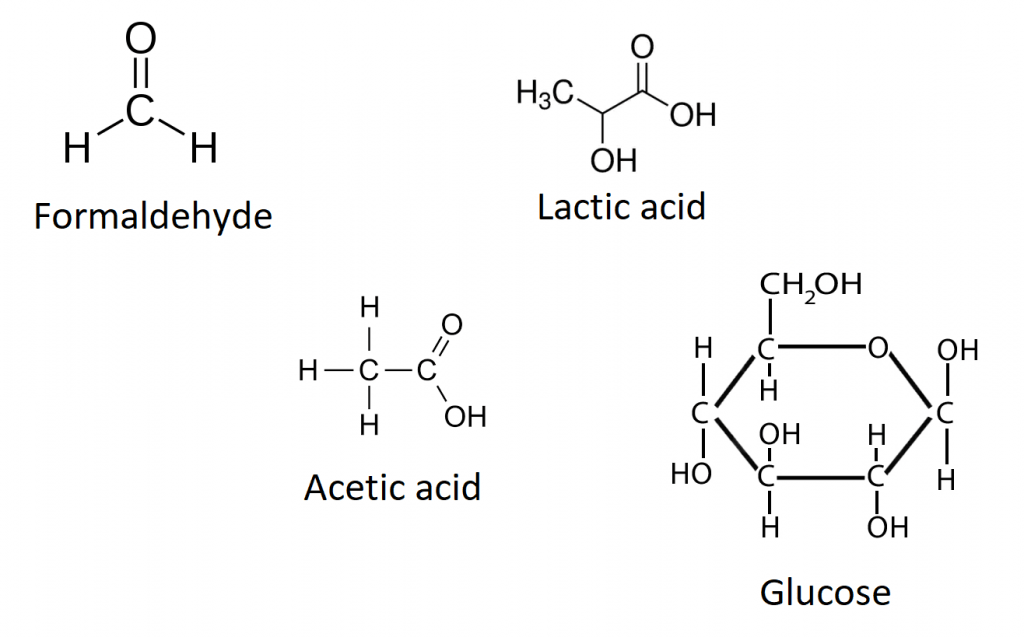
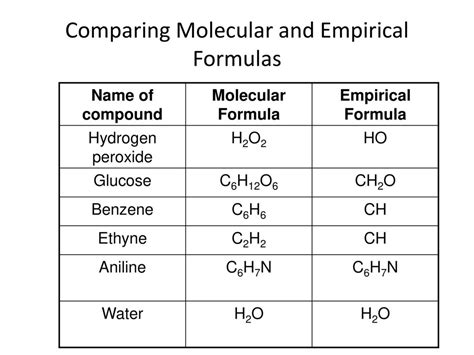
A molecular formula is a type of chemical formula that provides the exact number of atoms of each element present in a molecule. It is the most detailed level of description for the composition of a molecule, offering a complete picture of how many atoms of each element are chemically bonded together. For instance, the molecular formula of glucose is C6H12O6, indicating that each molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Molecular formulas are crucial in chemistry because they allow for the precise identification of substances, the calculation of molecular weights, and the determination of chemical properties.
Significance of Molecular Formulas
The significance of molecular formulas extends beyond the simple enumeration of atoms within a molecule. They are essential for understanding the chemical and physical properties of substances, as these properties are directly influenced by the molecular structure. Moreover, molecular formulas play a critical role in chemical reactions, as they help predict the quantities of reactants needed and products formed. The application of molecular formulas is not limited to chemistry; they are also vital in fields such as pharmacology, where the molecular structure of a drug can determine its efficacy and safety.
Empirical Formulas: The Simplest Representation
An empirical formula, on the other hand, represents the simplest whole-number ratio of atoms of each element present in a compound. Unlike molecular formulas, empirical formulas do not necessarily provide the actual number of atoms of each element in a molecule but rather the proportion in which they are present. For example, the empirical formula of glucose is CH2O, which indicates that for every carbon atom, there are two hydrogen atoms and one oxygen atom. Empirical formulas are derived from the molecular formula by dividing each of the subscripts by the greatest common divisor (GCD) of the subscripts.
Applications of Empirical Formulas
Empirical formulas have several practical applications in chemistry. They are useful for comparing the compositions of different compounds and for determining the chemical properties of substances. Since empirical formulas represent the simplest ratio of atoms, they can be used to identify compounds that have the same composition but differ in the number of formula units in their molecules. This is particularly useful in the study of polymers and other macromolecules, where the molecular weight can vary widely but the empirical formula remains constant.
| Formula Type | Description | Example |
|---|---|---|
| Molecular Formula | Exact number of atoms of each element | C6H12O6 (Glucose) |
| Empirical Formula | Simplest whole-number ratio of atoms | CH2O (Glucose) |
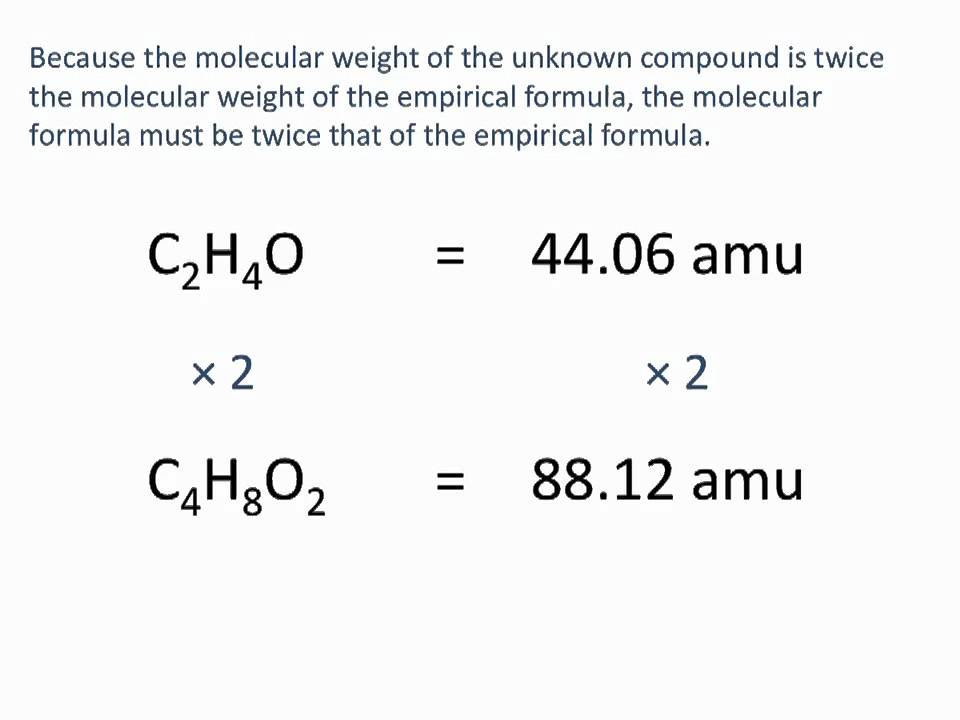
Determining Molecular Formulas from Empirical Formulas
Determining the molecular formula of a compound from its empirical formula requires additional information, typically the molecular weight of the compound. The process involves dividing the molecular weight by the formula weight (the sum of the atomic weights of the atoms in the empirical formula) to find the multiplier (n) that relates the empirical formula to the molecular formula. This calculation is essential in identifying the exact composition of a molecule, which is critical in various chemical and pharmacological applications.
Challenges and Considerations
While molecular and empirical formulas are indispensable tools in chemistry, there are challenges and considerations associated with their determination and application. For instance, the calculation of molecular formulas from empirical formulas can be complicated by the presence of isomers—compounds with the same molecular formula but different structural formulas. Moreover, the interpretation of empirical formulas requires a deep understanding of chemical bonding and the principles of stoichiometry.
What is the primary difference between molecular and empirical formulas?
+The primary difference is that molecular formulas provide the exact number of atoms of each element in a molecule, while empirical formulas provide the simplest whole-number ratio of atoms of each element.
How are empirical formulas derived from molecular formulas?
+Empirical formulas are derived by dividing each of the subscripts in the molecular formula by the greatest common divisor (GCD) of the subscripts.
What information is required to determine a molecular formula from an empirical formula?
+The molecular weight of the compound is required. The molecular formula can be determined by dividing the molecular weight by the formula weight of the empirical formula.
In conclusion, the distinction between molecular and empirical formulas is a fundamental concept in chemistry, each serving unique and complementary roles in understanding the composition and properties of chemical compounds. While molecular formulas provide detailed information about the exact number of atoms in a molecule, empirical formulas offer insights into the simplest ratio of atoms, facilitating comparisons and predictions of chemical behavior. The ability to determine and apply both types of formulas is essential for chemists, researchers, and students alike, as it underpins a wide range of chemical, pharmaceutical, and industrial applications.