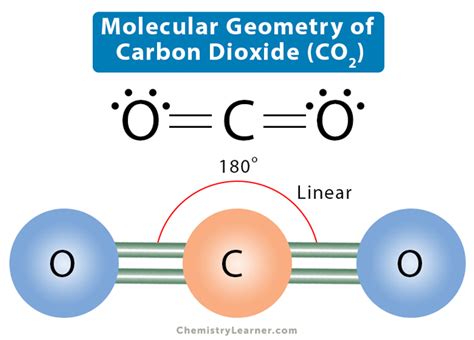
The molecular shape of carbon dioxide (CO2) is a fundamental concept in chemistry, crucial for understanding its physical and chemical properties. CO2 is a linear molecule, consisting of one carbon atom bonded to two oxygen atoms through double covalent bonds. This linear geometry is a result of the molecule's molecular orbital configuration and the nature of the chemical bonds between the atoms.
The carbon atom in CO2 is sp-hybridized, meaning that its electronic configuration is a mix of s and p orbitals, resulting in two hybrid orbitals that are oriented 180 degrees apart. This orientation leads to the formation of two double bonds between the carbon and oxygen atoms, with each oxygen atom also having two lone pairs of electrons. The combination of these double bonds and the lone pairs on the oxygen atoms results in a linear molecular shape, with the carbon atom at the center and the two oxygen atoms at opposite ends.
Molecular Geometry and Bonding
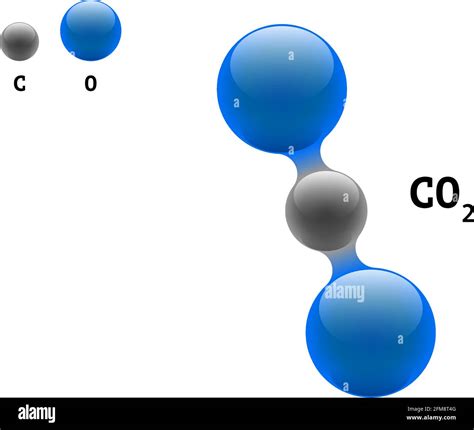
The molecular geometry of CO2 can be understood through the application of VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs around a central atom will arrange themselves to minimize repulsions. In the case of CO2, the two double bonds and the lack of lone pairs on the central carbon atom result in a linear arrangement of the atoms, with the bond angle being 180 degrees. This linear shape minimizes the repulsion between the electron pairs in the double bonds, leading to a stable molecular configuration.
The bonding in CO2 is characterized by the formation of sigma (σ) and pi (π) bonds between the carbon and oxygen atoms. Each double bond consists of one σ bond and one π bond, with the σ bonds resulting from the overlap of hybrid orbitals and the π bonds resulting from the overlap of unhybridized p orbitals. The presence of these double bonds gives CO2 its linear shape and contributes to its stability and reactivity.
Physical Properties and Chemical Reactivity
The linear molecular shape of CO2 has significant implications for its physical properties and chemical reactivity. CO2 is a non-polar molecule due to its symmetrical shape, resulting in its low solubility in water and high solubility in non-polar solvents. The molecule’s linear shape also influences its boiling and melting points, with CO2 sublimating (changing directly from a solid to a gas) at standard atmospheric pressure.
In terms of chemical reactivity, the linear shape of CO2 makes it susceptible to nucleophilic attack, particularly at the carbon atom. This reactivity is crucial in various chemical reactions, including the formation of carbonic acid (H2CO3) when CO2 reacts with water. The double bonds in CO2 also participate in addition reactions, where the molecule acts as an electrophile, accepting electron pairs from nucleophiles to form new bonds.
| Property | Value |
|---|---|
| Molecular Formula | CO2 |
| Molecular Weight | 44.01 g/mol |
| Boiling Point | -56.6°C (sublimes) |
| Melting Point | -78.5°C |
| Solubility in Water | 1.45 g/L at 25°C |
Key Points
- The molecular shape of CO2 is linear, resulting from the sp-hybridization of the carbon atom and the formation of double covalent bonds with oxygen atoms.
- The linear shape is stabilized by the arrangement of electron pairs that minimizes repulsions, as predicted by VSEPR theory.
- CO2's molecular shape influences its physical properties, including its non-polarity, solubility, and phase transition behavior.
- The linear shape and double bonds in CO2 contribute to its chemical reactivity, particularly in nucleophilic substitution and addition reactions.
- Understanding the molecular shape of CO2 is essential for appreciating its environmental and biological roles, including its function as a greenhouse gas and its participation in photosynthesis.
The molecular shape of CO2 is a fascinating example of how the arrangement of atoms within a molecule can influence its properties and behavior. Through the application of fundamental chemical principles, such as VSEPR theory and molecular orbital theory, we can gain a deeper understanding of the linear shape of CO2 and its implications for various chemical and physical processes.
Further exploration of CO2's molecular shape and its consequences can provide insights into the molecule's role in environmental and biological systems. The study of CO2's molecular geometry also underscores the importance of basic chemical principles in understanding complex phenomena, highlighting the value of interdisciplinary approaches in advancing our knowledge of the natural world.
What is the molecular shape of CO2, and why is it linear?
+The molecular shape of CO2 is linear due to the sp-hybridization of the carbon atom and the formation of double covalent bonds with the oxygen atoms, which results in a symmetrical arrangement of electron pairs that minimizes repulsions.
How does the molecular shape of CO2 influence its physical properties?
+The linear molecular shape of CO2 contributes to its non-polarity, low solubility in water, and high solubility in non-polar solvents. It also affects its boiling and melting points, with CO2 sublimating at standard atmospheric pressure.
What role does the molecular shape of CO2 play in its chemical reactivity?
+The linear shape and double bonds in CO2 make it susceptible to nucleophilic attack and participate in addition reactions, where the molecule acts as an electrophile. This reactivity is crucial in various chemical reactions, including the formation of carbonic acid.