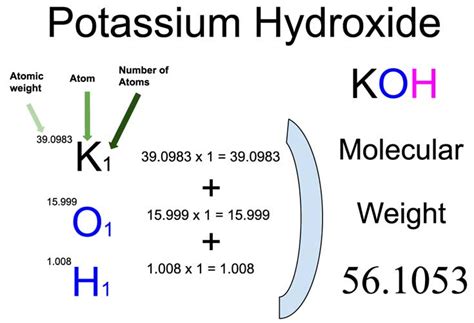
The molar mass of a chemical compound is a fundamental concept in chemistry, representing the total mass of a mole of that compound. For potassium hydroxide (KOH), a strong base commonly used in various industrial and laboratory applications, understanding its molar mass is crucial for precise calculations in chemical reactions and formulations. Potassium hydroxide is composed of one potassium (K) atom, one oxygen (O) atom, and one hydrogen (H) atom.
To calculate the molar mass of potassium hydroxide, we need to sum the atomic masses of its constituent atoms. The atomic masses (rounded to the nearest whole number for simplicity, but actual calculations use more precise values) are approximately: potassium (K) = 39 g/mol, oxygen (O) = 16 g/mol, and hydrogen (H) = 1 g/mol. Using these values, the molar mass of KOH can be calculated as follows: 39 (for K) + 16 (for O) + 1 (for H) = 56 g/mol.
Key Points
- The molar mass of potassium hydroxide (KOH) is calculated by adding the atomic masses of potassium, oxygen, and hydrogen.
- The atomic masses used for the calculation are approximately 39 g/mol for potassium, 16 g/mol for oxygen, and 1 g/mol for hydrogen.
- The calculated molar mass of KOH is 56 g/mol, which is essential for chemical calculations and reactions involving this compound.
- Potassium hydroxide is a strong base with various applications, including in the manufacture of soap, glass, and paper.
- Understanding the molar mass of compounds like KOH is vital for preparing solutions with precise concentrations, which is critical in both laboratory settings and industrial processes.
Chemical Properties and Applications of Potassium Hydroxide
Potassium hydroxide, with its strong basic properties, plays a significant role in various chemical reactions and industrial applications. It is highly soluble in water, producing a strong alkaline solution. This characteristic makes KOH useful in the production of soaps, where it acts as a saponification agent, turning fats and oils into soap and glycerol. Additionally, KOH is used in the manufacture of glass, where it helps in the melting and mixing of silica and other components, and in the paper industry, for pulping wood.
Calculations Involving Molar Mass of KOH
In chemical reactions and formulations, the molar mass of KOH is essential for calculating the amounts of reactants needed or the products formed. For instance, in the production of soap, knowing the molar mass of KOH allows manufacturers to calculate the exact amount of KOH required to react with a given amount of fat or oil, ensuring the production of high-quality soap with the desired properties. Similarly, in laboratory settings, the molar mass of KOH is crucial for preparing solutions of precise concentrations, which is vital for the accuracy and reliability of experimental results.
| Element | Atomic Mass (g/mol) | Contribution to KOH Molar Mass |
|---|---|---|
| Potassium (K) | 39.0983 | 39.0983 g/mol |
| Oxygen (O) | 15.9994 | 15.9994 g/mol |
| Hydrogen (H) | 1.00794 | 1.00794 g/mol |
| Total Molar Mass of KOH | 56.1057 g/mol |
Importance of Precision in Chemical Calculations

Precision in calculating the molar mass of compounds like potassium hydroxide is not just a matter of academic interest but has significant practical implications. In industrial processes, such as the manufacture of soap or glass, the exact concentration of KOH can affect the quality and consistency of the final product. Similarly, in laboratory experiments, precise control over the concentrations of reactants is essential for obtaining reliable and reproducible results. The molar mass of KOH, therefore, serves as a critical piece of information that underpins a wide range of chemical and industrial applications.
Evolution of Understanding Molar Mass
The concept of molar mass has evolved significantly over time, from the early understanding of atomic theory to the precise measurements of atomic masses today. The development of more accurate methods for determining atomic masses has allowed for the refinement of molar mass calculations for compounds like KOH. This evolution underscores the dynamic nature of scientific knowledge and the importance of continually refining our understanding of fundamental principles like molar mass.
What is the molar mass of potassium hydroxide (KOH)?
+The molar mass of KOH is approximately 56.1057 g/mol, calculated by summing the atomic masses of potassium, oxygen, and hydrogen.
Why is the molar mass of KOH important in chemical applications?
+The molar mass of KOH is crucial for calculating the amounts of reactants needed or products formed in chemical reactions, ensuring precise control over reaction conditions and product quality.
How does the precision of molar mass affect industrial applications?
+Precision in molar mass calculations affects the quality and consistency of products in industries like soap and glass manufacturing, where small variations in KOH concentration can have significant effects.



