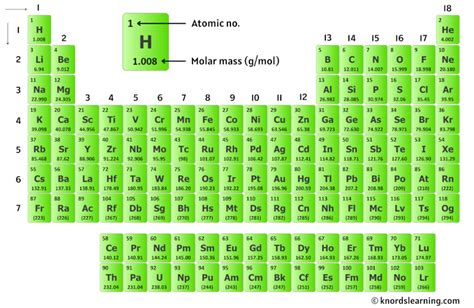
Calculating the molar mass of a compound is a fundamental concept in chemistry, crucial for understanding chemical reactions, stoichiometry, and the properties of substances. The molar mass, also known as molecular weight, is the sum of the atomic masses of all atoms in a molecule. It is expressed in units of grams per mole (g/mol). Mastering the calculation of molar mass is essential for chemists, researchers, and students alike. Here, we delve into five key tips for accurately determining the molar mass of compounds, exploring both the theoretical foundations and practical applications.
Understanding Atomic Masses
The first step in calculating the molar mass of a compound is to understand the atomic masses of its constituent elements. The atomic mass of an element is the average mass of one atom of that element and is usually expressed in atomic mass units (amu). However, for molar mass calculations, we use the atomic masses in grams per mole. These values can be found on the periodic table. It’s crucial to use the most accurate and up-to-date atomic masses, as these values can slightly vary due to the existence of isotopes. For instance, the atomic mass of carbon is approximately 12.01 g/mol, and that of oxygen is about 16.00 g/mol.
Breaking Down Compounds into Elements
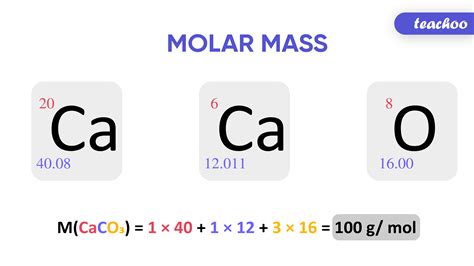
To calculate the molar mass of a compound, one must first identify all the elements present and their respective quantities as indicated by the chemical formula. For example, in the compound water (H2O), there are two hydrogen atoms and one oxygen atom. The molar mass of water would thus be calculated as (2 * atomic mass of hydrogen) + (1 * atomic mass of oxygen). Given that the atomic mass of hydrogen is approximately 1.008 g/mol, the calculation would be (2 * 1.008) + 16.00 = 18.016 g/mol.
| Element | Atomic Mass (g/mol) |
|---|---|
| Hydrogen (H) | 1.008 |
| Carbon (C) | 12.01 |
| Oxygen (O) | 16.00 |
| Nitrogen (N) | 14.01 |
Handling Parentheses and Multiplying Factors
In chemical formulas, parentheses are used to group atoms or polyatomic ions together, and multiplying factors (subscripts or coefficients) outside the parentheses indicate how many times the grouped atoms or ions are included in the compound. For instance, the formula for ammonium phosphate is (NH4)3PO4. Here, the parentheses around NH4 indicate a group that is repeated three times, as shown by the subscript 3 outside the parentheses. The calculation of the molar mass would involve multiplying the atomic masses of nitrogen, hydrogen, phosphorus, and oxygen by their respective quantities and then summing these values.
Calculating Molar Mass of Complex Compounds
For complex compounds, especially those involving polyatomic ions, it’s crucial to break down the compound into its simplest components and calculate the molar mass of each component before summing them. For example, in the case of sodium nitrate (NaNO3), we calculate the molar mass by adding the molar mass of sodium (approximately 22.99 g/mol), nitrogen (14.01 g/mol), and three times the molar mass of oxygen (3 * 16.00 g/mol = 48.00 g/mol), resulting in a total molar mass of 22.99 + 14.01 + 48.00 = 85.00 g/mol.
Key Points
- Understanding the atomic masses of elements is fundamental to calculating the molar mass of compounds.
- Accurately interpreting the chemical formula, including any parentheses or multiplying factors, is crucial.
- Molar mass calculations for complex compounds involve breaking down the compound into its simplest components.
- Using the most current and precise atomic masses ensures accuracy in molar mass calculations.
- Practicing with various types of compounds, including those with polyatomic ions, enhances understanding and skill.
In conclusion, calculating the molar mass of compounds requires a thorough understanding of atomic masses, the ability to interpret chemical formulas accurately, and attention to detail, especially when dealing with complex compounds or polyatomic ions. By mastering these skills and applying them consistently, one can accurately determine the molar mass of any compound, facilitating further chemical calculations and analyses.
What is the importance of using the most accurate atomic masses in molar mass calculations?
+Using the most accurate atomic masses is crucial because it ensures the precision of the molar mass calculation. Even small variations in atomic masses can lead to significant differences in the calculated molar mass of a compound, especially in compounds with a large number of atoms.
How do you handle compounds with parentheses in their chemical formulas?
+When a compound’s formula includes parentheses, you must multiply the atomic masses of all atoms within the parentheses by the subscript outside the parentheses. This ensures that you account for the correct number of each type of atom in the compound.
What is the difference between atomic mass and molar mass?
+Atomic mass refers to the mass of a single atom of an element, while molar mass refers to the mass of one mole of atoms or molecules of a substance. The molar mass is essentially the sum of the atomic masses of all atoms in a molecule, expressed in grams per mole (g/mol).