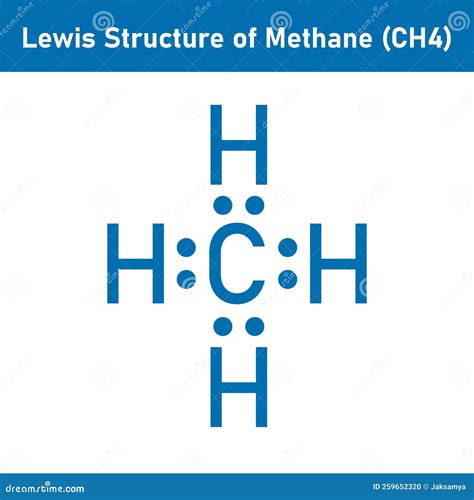
Methane, with the chemical formula CH₄, is one of the simplest organic compounds, consisting of a central carbon atom bonded to four hydrogen atoms. Understanding the Lewis structure of methane is crucial for comprehending its chemical properties and behavior. The Lewis structure, also known as the electron dot structure, is a simplified representation of the valence electrons in a molecule. Here, we will explore the process of drawing the methane Lewis structure and its implications.
Introduction to Lewis Structures
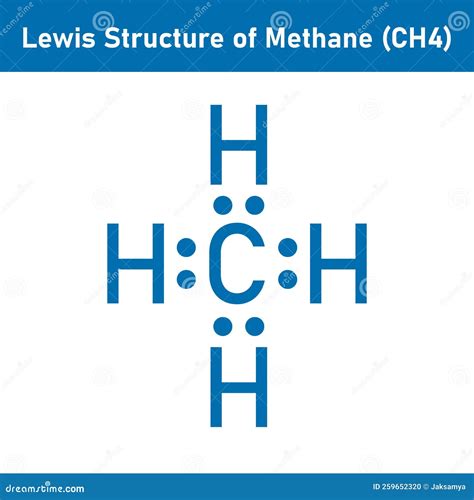
Lewis structures are a way to represent the covalent bonds between atoms in a molecule using dots to signify electrons. The process involves counting the total valence electrons in the molecule, drawing single bonds between the atoms, and then distributing the remaining electrons to satisfy the octet rule for each atom, except for hydrogen which requires two electrons to fill its 1s orbital. The Lewis structure of methane is a fundamental concept in organic chemistry, providing insights into its stability and reactivity.
Step 1: Counting Valence Electrons
The first step in drawing the Lewis structure of methane is to count the total number of valence electrons. Carbon has four valence electrons, and each hydrogen atom has one valence electron. Since methane has one carbon atom and four hydrogen atoms, the total number of valence electrons is calculated as follows: 4 (from carbon) + 4*1 (from the four hydrogen atoms) = 8 valence electrons.
Step 2: Drawing Single Bonds
Next, single bonds are drawn between the carbon atom and each of the four hydrogen atoms. Each single bond represents two shared electrons, which means 4 single bonds use 8 electrons (2 electrons per bond). However, in the case of methane, this step is simplified because we know the molecule’s structure is tetrahedral, with the carbon at the center and the hydrogens at the corners.
Step 3: Distributing Remaining Electrons
Given that the single bonds between carbon and hydrogen in methane use up all the available valence electrons (8 electrons for 4 single bonds), there are no remaining electrons to distribute. The carbon atom achieves an octet (8 electrons in its outer shell), and each hydrogen atom has two electrons, fulfilling the duet rule for hydrogen. This arrangement satisfies the basic requirements for a stable molecule according to Lewis theory.
Understanding the Methane Lewis Structure
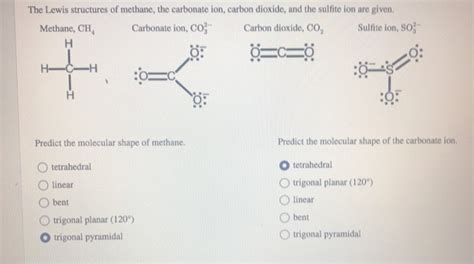
The methane Lewis structure shows a central carbon atom surrounded by four hydrogen atoms, each bonded to the carbon through a single covalent bond. This structure is significant because it illustrates the tetrahedral geometry of methane, where the carbon atom is sp³ hybridized. The tetrahedral shape of methane arises from the arrangement of its electron pairs (and thus its bonds) to minimize repulsion, leading to the most stable configuration.
Implications of the Methane Lewis Structure
The Lewis structure of methane has several implications for its chemical properties. The symmetrical, tetrahedral arrangement of atoms around the carbon atom results in a non-polar molecule, as the individual bond dipoles cancel each other out. This non-polarity influences methane’s physical properties, such as its relatively low boiling point and insolubility in water. Furthermore, the stability of the methane molecule, as inferred from its Lewis structure, contributes to its role as a fundamental building block in organic chemistry.
Key Points
- The methane molecule consists of one carbon atom bonded to four hydrogen atoms, forming a tetrahedral shape.
- The Lewis structure of methane is drawn by counting valence electrons, forming single bonds between atoms, and distributing remaining electrons to satisfy the octet rule.
- Methane's Lewis structure indicates a non-polar molecule due to the symmetrical arrangement of its bonds.
- The stability of methane, as shown by its Lewis structure, is crucial for its role in organic chemistry.
- Understanding the Lewis structure of methane is essential for predicting its chemical behavior and properties.
In conclusion, the Lewis structure of methane provides a foundational understanding of its chemical properties and behavior. By analyzing the steps involved in drawing the Lewis structure and understanding its implications, we gain insights into the molecular geometry, polarity, and stability of methane, which are crucial concepts in organic chemistry.
What is the significance of the Lewis structure in understanding methane’s properties?
+The Lewis structure is significant because it illustrates the arrangement of electrons in the molecule, which in turn influences its shape, polarity, and reactivity. For methane, the Lewis structure shows a tetrahedral geometry and a non-polar nature, which are key to understanding its physical and chemical properties.
How does the tetrahedral shape of methane arise from its Lewis structure?
+The tetrahedral shape arises from the sp³ hybridization of the carbon atom, which allows it to form four equivalent bonds with hydrogen atoms. This arrangement minimizes electron pair repulsion, resulting in a stable, three-dimensional shape where the carbon atom is at the center and the hydrogen atoms are at the corners of a tetrahedron.
What are the implications of methane being a non-polar molecule as indicated by its Lewis structure?
+The non-polarity of methane, as indicated by its symmetrical Lewis structure, implies that it has a low boiling point and is insoluble in water. Non-polar molecules tend to have weaker intermolecular forces compared to polar molecules, which affects their physical properties such as melting and boiling points, and their solubility in water.