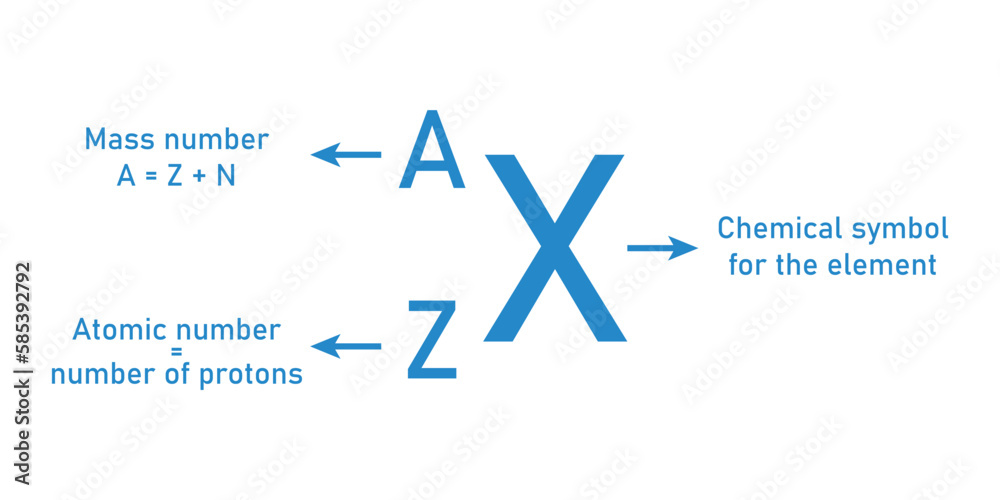
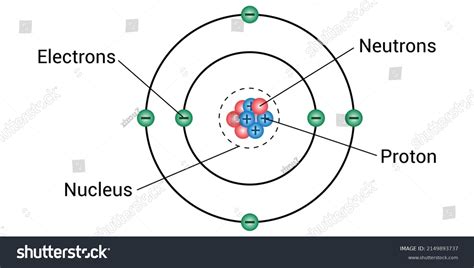
The atomic number of an element is defined by the number of protons present in the nucleus of an atom. In the case of an atom with 7 protons, we are referring to the element Nitrogen. Nitrogen is a chemical element with the symbol N and atomic number 7, which means that every nitrogen atom has 7 protons in its nucleus. This fundamental aspect of nitrogen's structure plays a critical role in its chemical properties and behaviors.
Chemical Properties of Nitrogen
Nitrogen’s chemical properties are largely influenced by its electron configuration, which is 1s² 2s² 2p³. The presence of 7 protons in the nucleus of a nitrogen atom results in a strong nuclear charge, which in turn affects the atom’s ability to attract electrons. Nitrogen is a nonmetal and is located in group 15 of the periodic table, also known as the pnictogens. It is the lightest member of this group and has a wide range of applications due to its unique chemical properties.
Nitrogen in Nature and Industry
Nitrogen is the fifth most abundant element in the universe by mass and makes up about 78% of the Earth’s atmosphere. Despite its abundance, nitrogen is often considered a limiting nutrient in many ecosystems because most organisms cannot use the nitrogen gas (N₂) that makes up the majority of the atmosphere. Instead, they require nitrogen in a more reactive form, such as ammonia (NH₃) or nitrate (NO₃⁻). The process of converting atmospheric nitrogen into these more usable forms is known as nitrogen fixation and is crucial for life on Earth.
| Form of Nitrogen | Chemical Formula | Usage |
|---|---|---|
| Nitrogen Gas | N₂ | Lighting, lasers, and as a component of the atmosphere |
| Ammonia | NH₃ | Fertilizers, cleaning products, and pharmaceuticals |
| Nitrate | NO₃⁻ | Fertilizers, explosives, and as a food additive |
Key Points
- Nitrogen has 7 protons in its atomic nucleus, defining its chemical properties and behaviors.
- The element is a nonmetal, located in group 15 of the periodic table, with a wide range of applications.
- Nitrogen is abundant in the Earth's atmosphere but is often a limiting nutrient due to its unreactive form (N₂).
- Nitrogen fixation, the process of converting N₂ into more reactive forms like NH₃ or NO₃⁻, is crucial for life and agriculture.
- The chemical properties of nitrogen, influenced by its electron configuration, play a critical role in its industrial applications and biological importance.
Biological Role of Nitrogen

Nitrogen is a fundamental component of amino acids, which are the building blocks of proteins. Proteins are essential for the structure, function, and regulation of the body’s tissues and organs. Nitrogen is also a key component of nucleic acids (DNA and RNA), which contain the genetic instructions used in the development and function of all living organisms. The cycle of nitrogen through the environment, involving processes like nitrogen fixation, ammonification, nitrification, and denitrification, is critical for maintaining the balance of ecosystems and supporting life on Earth.
Nitrogen Fixation and the Environment
Nitrogen fixation is the process by which nitrogen (N₂) from the atmosphere is converted into forms that can be used by living organisms, such as ammonia (NH₃) or nitrate (NO₃⁻). This process can occur through biological means, involving certain bacteria and algae, or through industrial processes, such as the Haber-Bosch process. While nitrogen fixation is essential for agriculture and food production, excessive nitrogen in the environment can lead to pollution, contributing to issues like eutrophication in water bodies and the formation of ground-level ozone, a component of smog.
Nitrogen's impact on the environment highlights the need for sustainable practices in agriculture and industry, aiming to minimize waste and prevent nitrogen pollution. Understanding the chemical properties of nitrogen and its role in ecosystems is crucial for developing strategies to manage nitrogen resources efficiently and reduce its environmental footprint.
What is the significance of nitrogen in the Earth's atmosphere?
+Nitrogen makes up about 78% of the Earth's atmosphere and is essential for life as it is a critical component of amino acids, nucleic acids, and chlorophyll. However, its unreactive form (N₂) means that most organisms cannot use it directly, making nitrogen fixation a vital process.
How does nitrogen fixation occur?
+Nitrogen fixation can occur through biological means, involving certain bacteria and algae, or through industrial processes like the Haber-Bosch process. Biological nitrogen fixation is a natural process that converts atmospheric nitrogen into ammonia or nitrate, which can be used by plants and other organisms.
What are the environmental impacts of excessive nitrogen?
+Excessive nitrogen in the environment can lead to pollution, contributing to issues like eutrophication in water bodies, where it promotes excessive algae growth, and the formation of ground-level ozone, a component of smog. Sustainable practices in agriculture and industry are needed to minimize nitrogen waste and prevent pollution.
In conclusion, the element with 7 protons, nitrogen, plays a vital role in both biological systems and industrial applications. Its unique chemical properties, abundance, and the challenge of utilizing its unreactive form (N₂) make it an essential element for life and a critical component of many industrial processes. Understanding nitrogen’s chemical properties and its environmental impacts is crucial for managing nitrogen resources sustainably and minimizing its footprint on the environment.