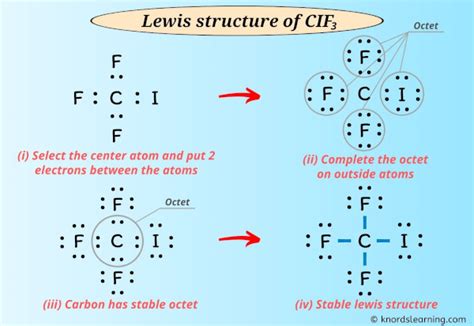
The CIF3 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of the CIF3 molecule, which consists of one chlorine atom bonded to three fluorine atoms. Understanding the Lewis structure is crucial for predicting the physical and chemical properties of the molecule. Here are five tips to help you draw and understand the CIF3 Lewis structure:
Key Points
- Determine the total number of valence electrons in the CIF3 molecule.
- Draw the skeletal structure, connecting the chlorine atom to the three fluorine atoms.
- Distribute the valence electrons to form covalent bonds and satisfy the octet rule for each atom.
- Predict the molecular geometry based on the VSEPR theory.
- Consider the polarity of the molecule and its implications on physical properties.
Understanding the CIF3 Molecule
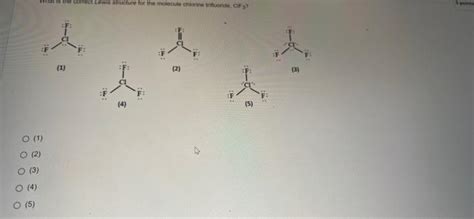
The CIF3 molecule is a type of interhalogen compound, which is formed by the reaction of chlorine with fluorine. To draw the Lewis structure, we first need to calculate the total number of valence electrons. Chlorine has 7 valence electrons, and each fluorine atom has 7 valence electrons. Therefore, the total number of valence electrons in CIF3 is 7 (Cl) + 3*7 (F) = 28 valence electrons.
Drawing the Skeletal Structure
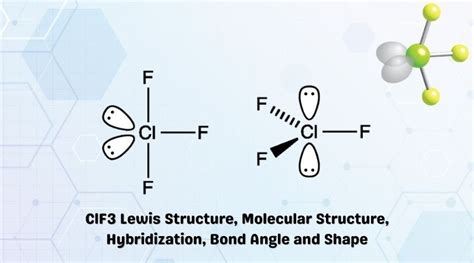
The skeletal structure of CIF3 can be drawn by connecting the chlorine atom to the three fluorine atoms. Since chlorine is the least electronegative atom, it will be the central atom. The three fluorine atoms will be bonded to the chlorine atom, forming a trigonal planar geometry.
| Atom | Valence Electrons | Role in CIF3 |
|---|---|---|
| Chlorine (Cl) | 7 | Central atom |
| Fluorine (F) | 7 | Peripheral atoms |
Distributing Valence Electrons

To distribute the valence electrons, we start by forming single covalent bonds between the chlorine atom and each fluorine atom. This uses 6 valence electrons, leaving 22 valence electrons remaining. The remaining electrons are distributed as lone pairs on the fluorine atoms and the chlorine atom, satisfying the octet rule for each atom.
Predicting Molecular Geometry
The molecular geometry of CIF3 can be predicted using the VSEPR theory. With three bonding pairs and two lone pairs around the central chlorine atom, the molecule adopts a trigonal planar geometry. However, due to the presence of lone pairs, the actual geometry is slightly distorted, resulting in a T-shaped or seesaw geometry.
Implications of Polarity
The CIF3 molecule is polar due to the difference in electronegativity between the chlorine and fluorine atoms. The polarity of the molecule affects its physical properties, such as its boiling point and solubility. Understanding the polarity of CIF3 is crucial for predicting its behavior in different chemical environments.
What is the total number of valence electrons in the CIF3 molecule?
+The total number of valence electrons in CIF3 is 28, calculated by adding the valence electrons of chlorine (7) and three fluorine atoms (3*7 = 21).
What is the molecular geometry of CIF3?
+The molecular geometry of CIF3 is trigonal planar, with the chlorine atom having three bonding pairs and two lone pairs. However, the actual geometry is slightly distorted due to the presence of lone pairs, resulting in a T-shaped or seesaw geometry.
Is the CIF3 molecule polar?
+Yes, the CIF3 molecule is polar due to the difference in electronegativity between the chlorine and fluorine atoms. This polarity affects its physical properties, such as its boiling point and solubility.
In conclusion, understanding the CIF3 Lewis structure is essential for predicting the physical and chemical properties of the molecule. By following the five tips outlined above, you can draw and understand the Lewis structure, predict the molecular geometry, and consider the polarity of the molecule. This knowledge is crucial for working with CIF3 in various chemical environments and applications.