The debate between lactated Ringer's solution and normal saline has been a longstanding one in the medical community, with each having its own set of advantages and disadvantages. Both solutions are widely used for fluid resuscitation, maintenance, and as a vehicle for drug administration. Understanding the differences between these two solutions is crucial for healthcare professionals to make informed decisions about patient care.
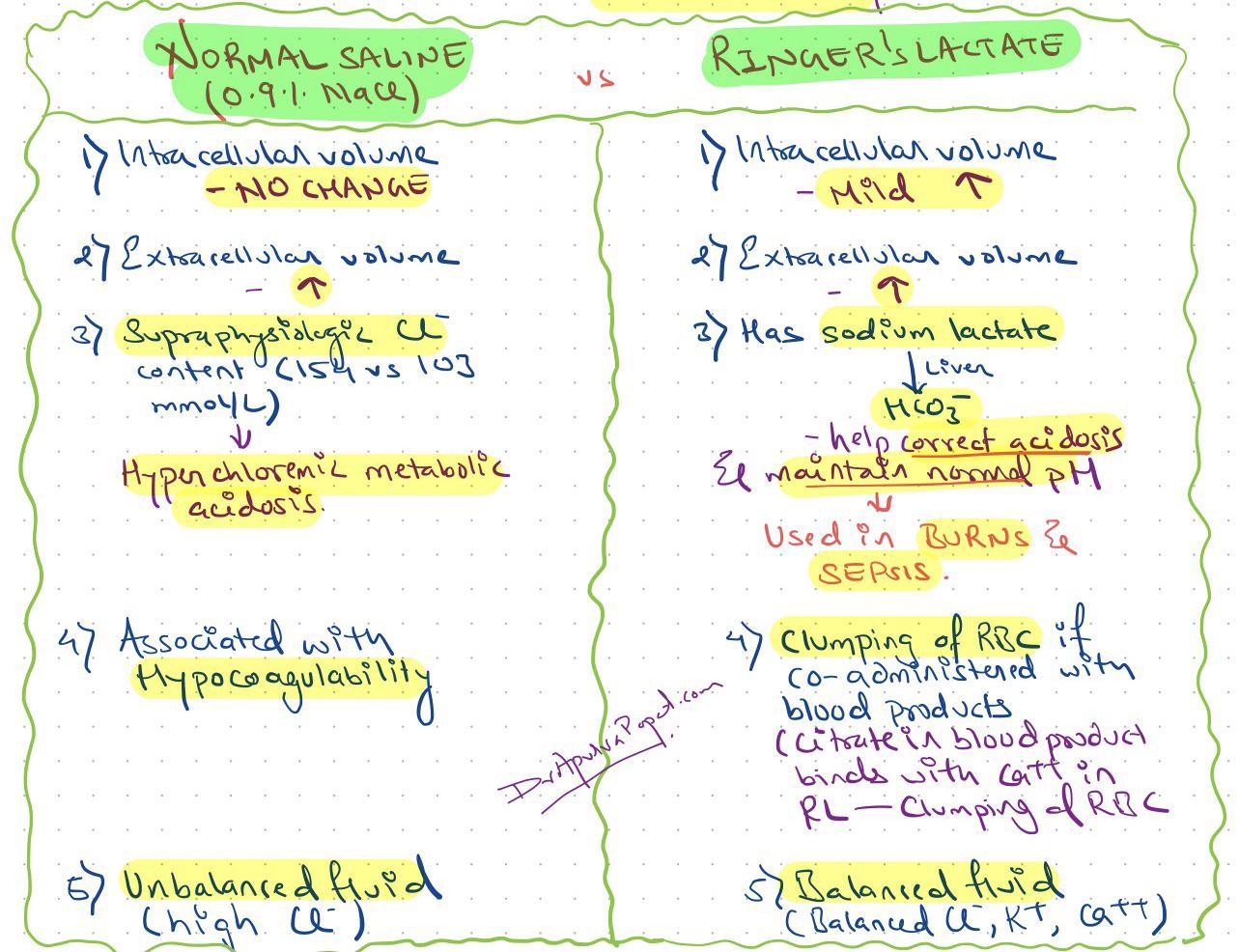
Lactated Ringer's solution, also known as Ringer's lactate, is a crystalloid fluid that contains sodium chloride, sodium lactate, potassium chloride, and calcium chloride. The composition of lactated Ringer's solution is designed to mimic the electrolyte composition of human plasma, with a pH range of 6.5-7.5. Normal saline, on the other hand, is a 0.9% solution of sodium chloride in water, with a pH range of 4.5-7.0. The primary difference between the two solutions lies in their electrolyte composition and pH levels.
Key Points
- Lactated Ringer's solution and normal saline are both used for fluid resuscitation and maintenance.
- Lactated Ringer's solution has a more balanced electrolyte composition compared to normal saline.
- Normal saline has a higher risk of causing hyperchloremic metabolic acidosis due to its high chloride content.
- Lactated Ringer's solution is more effective in correcting acid-base imbalances due to its lactate content.
- The choice between lactated Ringer's solution and normal saline depends on the individual patient's needs and underlying medical conditions.
Composition and pH Levels

The composition of lactated Ringer’s solution and normal saline differs significantly. Lactated Ringer’s solution contains 130 mEq/L of sodium, 109 mEq/L of chloride, 28 mEq/L of lactate, 4 mEq/L of potassium, and 3 mEq/L of calcium. In contrast, normal saline contains 154 mEq/L of sodium and 154 mEq/L of chloride, with no potassium or calcium. The lactate in lactated Ringer’s solution helps to buffer the pH and prevent metabolic acidosis.
The pH levels of the two solutions also differ. Lactated Ringer's solution has a pH range of 6.5-7.5, which is closer to the normal human plasma pH range of 7.35-7.45. Normal saline, on the other hand, has a pH range of 4.5-7.0, which is more acidic. The lower pH of normal saline can contribute to the development of hyperchloremic metabolic acidosis, particularly when administered in large volumes.
Clinical Implications
The choice between lactated Ringer’s solution and normal saline depends on the individual patient’s needs and underlying medical conditions. Lactated Ringer’s solution is generally preferred for fluid resuscitation and maintenance in patients with normal kidney function, as it provides a more balanced electrolyte composition and helps to correct acid-base imbalances. Normal saline, on the other hand, may be preferred in patients with severe hyperkalemia or those who require a high volume of fluid resuscitation, as it can help to dilute potassium levels and provide a rapid increase in intravascular volume.
However, the use of normal saline in large volumes can lead to hyperchloremic metabolic acidosis, which can have significant clinical implications. A study published in the Critical Care Medicine journal found that patients who received large volumes of normal saline had a higher incidence of metabolic acidosis and worse outcomes compared to those who received lactated Ringer's solution.
| Electrolyte Composition | Lactated Ringer's Solution | Normal Saline |
|---|---|---|
| Sodium (mEq/L) | 130 | 154 |
| Chloride (mEq/L) | 109 | 154 |
| Lactate (mEq/L) | 28 | 0 |
| Potassium (mEq/L) | 4 | 0 |
| Calcium (mEq/L) | 3 | 0 |

Conclusion and Future Directions
In conclusion, lactated Ringer’s solution and normal saline are both widely used crystalloid fluids with different compositions and pH levels. While lactated Ringer’s solution is generally preferred for fluid resuscitation and maintenance due to its more balanced electrolyte composition, normal saline may be preferred in specific clinical situations. Further research is needed to fully understand the clinical implications of each solution and to develop evidence-based guidelines for their use.
What is the primary difference between lactated Ringer's solution and normal saline?
+The primary difference between lactated Ringer's solution and normal saline lies in their electrolyte composition and pH levels. Lactated Ringer's solution contains a more balanced electrolyte composition, including sodium, chloride, lactate, potassium, and calcium, while normal saline contains only sodium and chloride.
What are the potential clinical implications of using normal saline in large volumes?
+The use of normal saline in large volumes can lead to hyperchloremic metabolic acidosis, which can have significant clinical implications, including increased morbidity and mortality. Additionally, normal saline can exacerbate existing acid-base imbalances and worsen patient outcomes.
What is the role of lactate in lactated Ringer's solution?
+The lactate in lactated Ringer's solution helps to buffer the pH and prevent metabolic acidosis. Lactate is metabolized by the liver and kidneys, producing bicarbonate, which helps to correct acid-base imbalances and maintain a normal pH.
Ultimately, the choice between lactated Ringer’s solution and normal saline depends on a thorough understanding of their composition, pH levels, and potential clinical implications. By carefully considering these factors, healthcare professionals can provide optimal patient care and improve outcomes in a variety of clinical settings.



