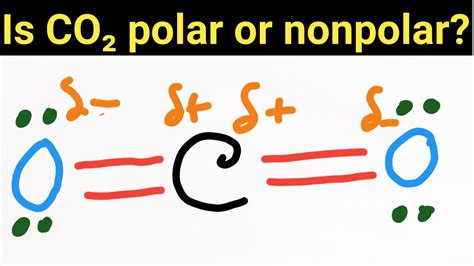
The question of whether CO2 (carbon dioxide) is polar or nonpolar is a fundamental concept in chemistry, particularly in understanding the properties and behaviors of molecules. To address this, we must first understand what it means for a molecule to be polar or nonpolar. A polar molecule is one that has a net dipole moment, meaning it has a slightly positive charge on one side and a slightly negative charge on the other. This occurs due to a difference in electronegativity between the atoms in the molecule, which leads to an unequal sharing of electrons. On the other hand, a nonpolar molecule has no net dipole moment, as the electrons are shared more equally between the atoms, resulting in no significant charge separation.
Understanding CO2 Molecular Structure
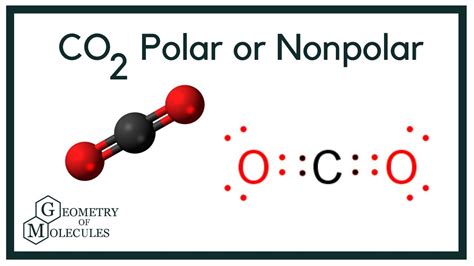
Carbon dioxide (CO2) is composed of one carbon atom and two oxygen atoms. The molecular structure of CO2 is linear, with the carbon atom in the center and the two oxygen atoms at either end. This linear geometry is crucial in determining the polarity of the molecule. In a linear molecule like CO2, the carbon atom is bonded to the two oxygen atoms through double covalent bonds. The electronegativity of oxygen is higher than that of carbon, which means oxygen has a greater tendency to attract electrons towards itself in a covalent bond.
Electronegativity and Bond Polarity
Although the bonds between carbon and oxygen in CO2 are polar due to the difference in electronegativity (oxygen being more electronegative than carbon), the overall molecule is nonpolar. This might seem counterintuitive at first, but it’s due to the symmetric distribution of the bond dipoles in the linear CO2 molecule. The dipole moments of the two C-O bonds are equal in magnitude and opposite in direction, which results in their vector sum being zero. This means that the molecule as a whole does not have a net dipole moment, and therefore, it is considered nonpolar.
| Atom | Electronegativity |
|---|---|
| Carbon (C) | 2.55 |
| Oxygen (O) | 3.44 |
Key Points
- CO2 is a linear molecule with a carbon atom bonded to two oxygen atoms through double covalent bonds.
- The bonds between carbon and oxygen are polar due to the difference in electronegativity.
- Despite the polarity of the C-O bonds, the CO2 molecule is nonpolar due to its symmetric linear structure.
- The electronegativity of oxygen (3.44) is higher than that of carbon (2.55), contributing to the polarity of the bonds.
- The nonpolarity of CO2 is a result of the vector sum of the bond dipoles being zero.
In conclusion, while the individual bonds in CO2 are polar, the molecule itself is nonpolar due to its linear, symmetric structure. This understanding is crucial for predicting the physical and chemical properties of CO2, such as its solubility in water and its behavior in different chemical reactions. The distinction between bond polarity and molecular polarity is fundamental in chemistry, and the case of CO2 serves as a clear example of how molecular geometry can influence the overall polarity of a molecule.
What makes a molecule polar or nonpolar?
+A molecule is polar if it has a net dipole moment, which occurs when there is a significant difference in electronegativity between the atoms in the molecule, leading to an unequal sharing of electrons. A molecule is nonpolar if it does not have a net dipole moment, either because the electrons are shared more equally or because the dipole moments of the bonds cancel each other out due to the molecular geometry.
Is the polarity of a molecule the same as the polarity of its bonds?
+No, the polarity of a molecule is not the same as the polarity of its bonds. While the polarity of bonds contributes to the overall polarity of a molecule, the molecular geometry plays a crucial role in determining whether the molecule is polar or nonpolar. A molecule can have polar bonds but still be nonpolar if the bond dipoles cancel each other out due to the molecular structure.
What role does molecular geometry play in determining the polarity of a molecule?
+Molecular geometry is critical in determining the polarity of a molecule. The shape of the molecule dictates how the dipole moments of the individual bonds interact with each other. In symmetric molecules like CO2, the bond dipoles can cancel each other out, resulting in a nonpolar molecule despite the presence of polar bonds. In asymmetric molecules, the bond dipoles do not cancel out, leading to a polar molecule.