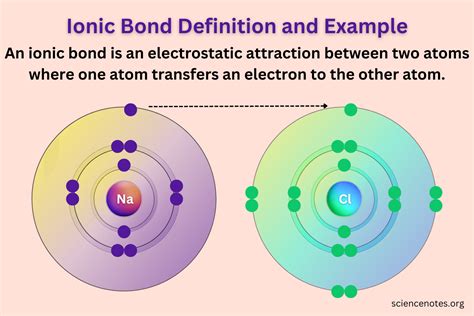
The formation of ionic bonds is a fundamental concept in chemistry, representing one of the primary ways in which atoms form chemical bonds. This type of bonding occurs when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges, which are then attracted to each other. The process of ionic bonding is crucial for understanding the structure and properties of numerous compounds, especially those involving metals and nonmetals. To delve into the specifics of ionic bonds, it's essential to explore some examples that illustrate how this type of bonding occurs and the characteristics of the compounds it forms.
Key Points
- Ionic bonds are formed through the transfer of electrons between atoms, leading to the creation of positively and negatively charged ions.
- The primary examples of ionic compounds include sodium chloride (NaCl), calcium carbonate (CaCO3), and magnesium oxide (MgO), which are commonly found in nature and have significant industrial applications.
- The properties of ionic compounds, such as their high melting points, brittleness, and solubility in water, are directly related to the strength of the ionic bonds and the arrangement of the ions in the crystal lattice.
- The formation of ionic bonds is often driven by the desire of atoms to achieve a stable electronic configuration, typically a noble gas configuration, which is characterized by a full outer shell of electrons.
- Understanding ionic bonding is crucial for predicting the properties and reactivity of ionic compounds, which play vital roles in various biological, geological, and industrial processes.
Formation of Ionic Bonds

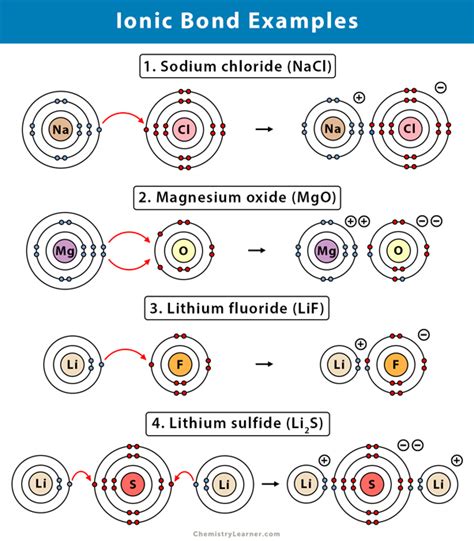
The process of forming an ionic bond involves the transfer of one or more electrons from a metal atom to a nonmetal atom. This electron transfer results in the metal atom becoming a positively charged ion (cation) and the nonmetal atom becoming a negatively charged ion (anion). The electrostatic attraction between the oppositely charged ions holds them together, forming a strong chemical bond. For instance, when sodium (Na) and chlorine (Cl) react, sodium loses an electron to become a positively charged sodium ion (Na+), while chlorine gains an electron to become a negatively charged chloride ion (Cl-). The resulting compound, sodium chloride (NaCl), is a classic example of an ionic compound.
Sodium Chloride (NaCl) as an Ionic Bond Example
Sodium chloride, commonly known as table salt, is one of the most recognizable ionic compounds. Its formation involves the reaction between sodium (a highly reactive metal) and chlorine (a highly reactive nonmetal), where each sodium atom donates an electron to a chlorine atom. This electron transfer leads to the formation of sodium ions (Na+) and chloride ions (Cl-), which then attract each other to form a crystalline solid. The structure of NaCl is characterized by a cubic lattice, where each sodium ion is surrounded by six chloride ions, and vice versa, demonstrating the strong ionic interactions that hold the compound together.
| Compound | Chemical Formula | Properties |
|---|---|---|
| Sodium Chloride | NaCl | High melting point, soluble in water, brittle |
| Calcium Carbonate | CaCO3 | Insoluble in water, decomposes at high temperatures, found in limestone and chalk |
| Magnesium Oxide | MgO | High melting point, insoluble in water, used in refractory applications |
Properties of Ionic Compounds
Ionic compounds exhibit a range of characteristic properties due to the nature of the ionic bonds that hold them together. These properties include high melting and boiling points, brittleness, and, in many cases, high solubility in water. The high melting points of ionic compounds are a direct result of the strong electrostatic attractions between the oppositely charged ions, which require a significant amount of energy to overcome. The brittleness of ionic compounds can be attributed to the rigid structure of the ionic lattice, which cannot easily deform without breaking. Furthermore, the solubility of ionic compounds in water is influenced by the ability of water molecules to interact with and separate the ions, facilitating their dissolution.
Calcium Carbonate and Magnesium Oxide as Examples
Beyond sodium chloride, other ionic compounds such as calcium carbonate (CaCO3) and magnesium oxide (MgO) demonstrate the diversity and importance of ionic bonding in nature. Calcium carbonate, found in limestone and chalk, is a key component of many biological structures, such as shells and skeletons, and plays a critical role in the carbon cycle. Magnesium oxide, with its high melting point and insolubility in water, is used in refractory applications and as a source of magnesium in various industrial processes. Both compounds illustrate how ionic bonds contribute to the formation of compounds with unique properties and applications.
What is the primary characteristic of ionic bonds?
+The primary characteristic of ionic bonds is the transfer of electrons between atoms, resulting in the formation of oppositely charged ions that are attracted to each other.
Why do ionic compounds typically have high melting points?
+Ionic compounds have high melting points due to the strong electrostatic attractions between the oppositely charged ions, which require a significant amount of energy to overcome.
What role do ionic compounds play in biological systems?
+Ionic compounds play critical roles in biological systems, including the formation of bones and shells, the regulation of fluid balance, and the facilitation of nerve impulses.
In conclusion, the study of ionic bonds and the compounds they form is a rich and complex field that underlies many aspects of chemistry, biology, and geology. By examining the formation, properties, and applications of ionic compounds, we gain insight into the fundamental principles that govern the behavior of matter at the atomic and molecular level. The diversity of ionic compounds, from the simplicity of sodium chloride to the complexity of biological minerals, underscores the importance of understanding ionic bonding in its various manifestations.