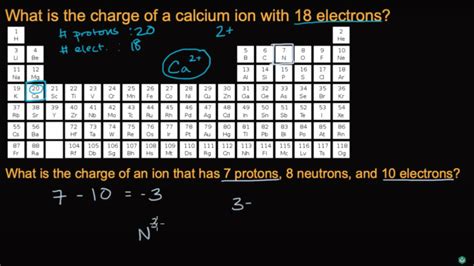
Calcium ions play a crucial role in various biological and chemical processes, and understanding their charge is essential for comprehending their behavior and interactions. Calcium, an alkaline earth metal, has an atomic number of 20 and an atomic mass of 40.078 u (unified atomic mass units). In its elemental form, calcium has a neutral charge, but when it loses or gains electrons, it becomes an ion with a specific charge.
The calcium ion, denoted as Ca²⁺, is a cation with a +2 charge. This charge arises from the loss of two electrons from the neutral calcium atom, resulting in a stable ion with a full outer energy level. The +2 charge of the calcium ion is a consequence of the atom's electronic configuration, which is [Ar] 4s². When calcium loses two electrons, it attains a noble gas configuration, becoming isoelectronic with argon (Ar).
Key Points
- The calcium ion has a +2 charge due to the loss of two electrons from the neutral atom.
- The electronic configuration of calcium is [Ar] 4s², leading to a stable ion with a full outer energy level.
- Calcium ions play a vital role in various biological processes, including muscle contraction, nerve function, and bone formation.
- The charge of the calcium ion affects its interactions with other ions and molecules, influencing its behavior in different environments.
- Understanding the charge of calcium ions is essential for applications in fields like medicine, agriculture, and materials science.
Calcium Ion Charge and Its Significance
The +2 charge of the calcium ion has significant implications for its behavior and interactions. In aqueous solutions, calcium ions are hydrated, forming complexes with water molecules. The charge of the calcium ion also affects its interactions with other ions, such as anions like chloride (Cl⁻) or phosphate (PO₄³⁻). These interactions are crucial in various biological processes, including muscle contraction, nerve function, and bone formation.
Biological Importance of Calcium Ions
Calcium ions play a vital role in many biological processes, including:
- Muscle contraction: Calcium ions trigger muscle contraction by binding to troponin and tropomyosin, allowing actin and myosin to interact.
- Nerve function: Calcium ions are involved in neurotransmitter release and synaptic plasticity, influencing learning and memory.
- Bone formation: Calcium ions are essential for bone mineralization, with hydroxyapatite (Ca₁₀(PO₄)₆(OH)₂) being the primary mineral component of bone tissue.
| Biological Process | Role of Calcium Ions |
|---|---|
| Muscle Contraction | Trigger contraction by binding to troponin and tropomyosin |
| Nerve Function | Involved in neurotransmitter release and synaptic plasticity |
| Bone Formation | Essential for bone mineralization, forming hydroxyapatite |
Applications of Calcium Ions
The unique properties of calcium ions make them useful in various applications, including:
- Medicine: Calcium ions are used in medical treatments, such as calcium channel blockers for hypertension and calcium supplements for osteoporosis.
- Agriculture: Calcium ions are essential for plant growth, influencing cell wall development and nutrient uptake.
- Materials Science: Calcium ions are used in the production of cement, concrete, and other construction materials.
Conclusion and Future Directions
In conclusion, the calcium ion charge is a fundamental aspect of its behavior and interactions. Understanding the +2 charge of the calcium ion is essential for comprehending its role in various biological and chemical processes. As research continues to uncover the complexities of calcium ion interactions, new applications and technologies are likely to emerge, further highlighting the importance of this versatile ion.
What is the charge of a calcium ion?
+The charge of a calcium ion is +2, resulting from the loss of two electrons from the neutral calcium atom.
Why are calcium ions important in biological processes?
+Calcium ions play a vital role in various biological processes, including muscle contraction, nerve function, and bone formation, due to their unique interactions with other molecules and ions.
What are some applications of calcium ions?
+Calcium ions have various applications in medicine, agriculture, and materials science, including medical treatments, plant growth, and construction materials.