Calculating percent yield is a fundamental concept in chemistry, particularly in the context of chemical reactions and synthesis. It is a measure of the efficiency of a reaction, indicating how much of the desired product is actually obtained compared to the theoretical maximum. Understanding percent yield is crucial for chemists and researchers as it helps in optimizing reaction conditions, identifying potential losses, and scaling up processes. In this article, we will delve into the concept of percent yield, its importance, and provide a step-by-step guide on how to calculate it easily.
Key Points
- Definition and importance of percent yield in chemical reactions
- Formula for calculating percent yield
- Step-by-step guide to calculating percent yield
- Factors affecting percent yield
- Practical applications of percent yield in chemistry and industry
Understanding Percent Yield
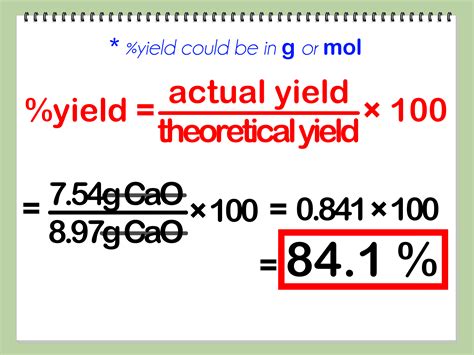
Percent yield, also known as percentage yield, is defined as the ratio of the actual yield (the amount of product actually obtained) to the theoretical yield (the amount of product that would be obtained if the reaction were 100% efficient), multiplied by 100. It is expressed as a percentage and provides a quantitative measure of the reaction’s efficiency. The closer the percent yield is to 100%, the more efficient the reaction is.
Calculating Percent Yield
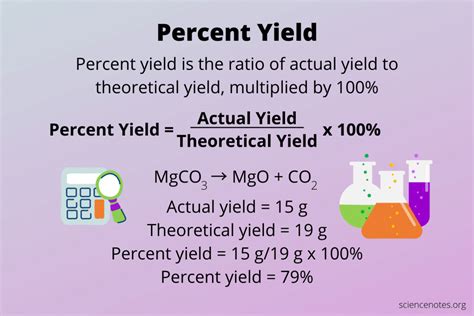
The formula for calculating percent yield is straightforward: Percent Yield = (Actual Yield / Theoretical Yield) * 100. The actual yield is the amount of product isolated after the reaction, while the theoretical yield is calculated based on the limiting reactant and the stoichiometry of the reaction.
| Component | Value |
|---|---|
| Actual Yield | Amount of product isolated |
| Theoretical Yield | Maximum amount of product possible based on reaction stoichiometry |
| Percent Yield Formula | (Actual Yield / Theoretical Yield) * 100 |
Factors Affecting Percent Yield

Several factors can influence the percent yield of a chemical reaction. These include the purity of the reactants, the reaction conditions such as temperature and pressure, the presence of catalysts or inhibitors, and the efficiency of the separation and purification processes. Understanding these factors is key to optimizing reaction conditions and improving the percent yield.
Practical Applications
Percent yield has significant practical applications in both research and industrial settings. It helps chemists to evaluate the efficiency of new synthetic routes, to compare different reaction conditions, and to scale up reactions from the laboratory to the industrial scale. In industry, optimizing percent yield can lead to cost savings, reduced waste, and more efficient use of resources.
What is the significance of calculating percent yield in chemical reactions?
+Calculating percent yield is significant because it measures the efficiency of a reaction, helping in the optimization of reaction conditions and the identification of potential losses.
How can percent yield be improved in a chemical reaction?
+Percent yield can be improved by optimizing reaction conditions such as temperature, pressure, and the use of catalysts, as well as ensuring the purity of reactants and the efficiency of product isolation and purification processes.
What are the implications of a low percent yield in industrial chemistry?
+A low percent yield in industrial chemistry can lead to increased costs, reduced profitability, and environmental concerns due to waste generation. Therefore, optimizing percent yield is crucial for sustainable and efficient industrial processes.
In conclusion, calculating percent yield is a critical aspect of chemical reactions, providing insights into reaction efficiency and helping in the optimization of conditions for better yields. By understanding the factors that affect percent yield and applying this knowledge in practice, chemists and industries can work towards more efficient, sustainable, and cost-effective chemical synthesis processes.