Understanding electron configuration is a fundamental aspect of chemistry, as it describes the distribution of electrons within an atom. This concept is crucial for understanding the chemical properties of elements and how they interact with each other. Electron configuration can seem complex at first, but with a systematic approach, it can be made easier to understand and determine.
Key Points
- The electron configuration of an atom describes the arrangement of its electrons in different energy levels or shells.
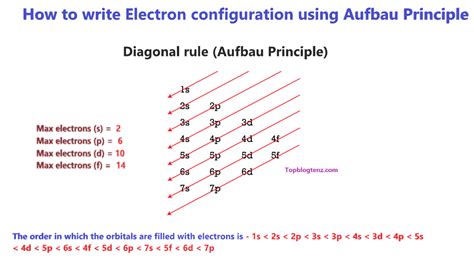
- Electrons are arranged in shells according to the Aufbau principle and the Pauli Exclusion Principle.
- The Aufbau principle states that electrons fill the lowest available energy levels first.
- The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers.
- Understanding electron configuration is essential for predicting the chemical behavior of elements.
Understanding the Basics of Electron Configuration
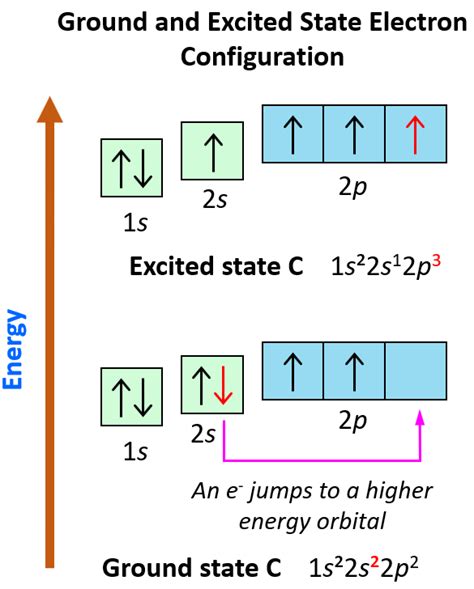
To find the electron configuration of an element, you need to know the atomic number of the element, which tells you the total number of electrons in a neutral atom. The electrons are arranged in different energy levels or shells around the nucleus. Each shell has a specific capacity for electrons, and the electrons in each shell are arranged in orbitals. The first shell (or 1s orbital) can hold up to 2 electrons, the second shell can hold up to 8 electrons (2s and 2p orbitals), the third shell can hold up to 18 electrons (3s, 3p, and 3d orbitals), and so on.
Applying the Aufbau Principle and Pauli Exclusion Principle
The Aufbau principle guides us in filling the lowest available energy levels first. Starting from the 1s orbital, we fill each orbital with the maximum number of electrons it can hold before moving on to the next. The Pauli Exclusion Principle ensures that each electron in an atom has a unique set of quantum numbers (n, l, m, and s), which means no two electrons can occupy the same orbital with the same spin.
| Shell | Orbitals | Maximum Electrons |
|---|---|---|
| 1st | 1s | 2 |
| 2nd | 2s, 2p | 8 |
| 3rd | 3s, 3p, 3d | 18 |
| 4th | 4s, 4p, 4d, 4f | 32 |
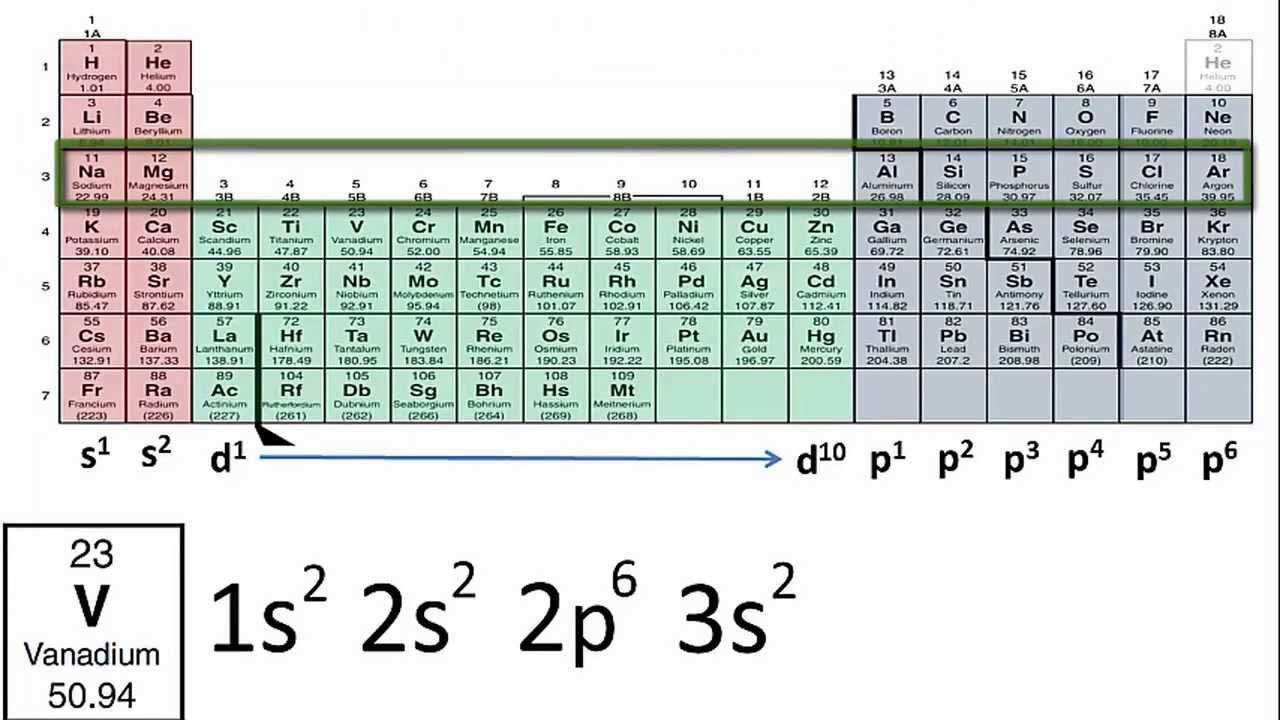
Practical Steps to Determine Electron Configuration
To determine the electron configuration of an element, follow these steps:
- Identify the atomic number of the element, which gives you the total number of electrons.
- Start filling the electrons into the lowest available energy levels, following the Aufbau principle.
- Apply the Pauli Exclusion Principle to ensure each electron has a unique set of quantum numbers.
- Continue filling electrons into the orbitals until all electrons are accounted for.
Example: Electron Configuration of Carbon
Carbon has an atomic number of 6, meaning it has 6 electrons. Following the Aufbau principle, the first 2 electrons fill the 1s orbital, and the next 4 electrons fill the 2s and 2p orbitals. Thus, the electron configuration of carbon is 1s² 2s² 2p².
Mastering electron configuration requires practice and a deep understanding of the underlying principles. By applying these principles and following a systematic approach, you can easily determine the electron configuration of any element and gain insights into its chemical properties and behavior.
What is the significance of electron configuration in chemistry?
+Electron configuration is significant because it helps predict the chemical behavior of elements, including their reactivity and the types of bonds they can form.
How does the Aufbau principle affect electron configuration?
+The Aufbau principle ensures that electrons fill the lowest available energy levels first, guiding the arrangement of electrons in an atom.
What is the role of the Pauli Exclusion Principle in electron configuration?
+The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers, ensuring each electron has a unique position and spin.
In conclusion, finding electron configuration easily requires a grasp of the fundamental principles that govern the arrangement of electrons in atoms. By understanding and applying the Aufbau principle and the Pauli Exclusion Principle, you can determine the electron configuration of any element and deepen your understanding of chemistry.