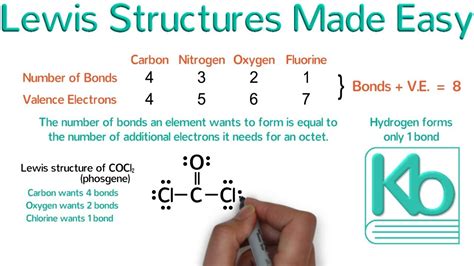
Learning to draw Lewis structures is a fundamental skill for chemistry students, as it provides a visual representation of the molecular structure and helps in understanding the chemical properties of a molecule. A Lewis structure, also known as an electron dot diagram, shows the arrangement of electrons in a molecule, highlighting the bonds between atoms and the lone pairs of electrons. Drawing Lewis structures can seem daunting at first, but with practice and a systematic approach, it becomes easier. In this article, we will outline the steps to draw Lewis structures easily, discuss the importance of Lewis structures in understanding molecular properties, and provide examples to illustrate the process.
Key Points
- Understand the basic rules for drawing Lewis structures, including counting valence electrons and applying the octet rule.
- Learn to identify the central atom in a molecule, which is crucial for determining the arrangement of atoms and electrons.
- Practice drawing Lewis structures for different types of molecules, including simple molecules like CO2 and more complex ones like benzene.
- Recognize the limitations of Lewis structures, such as their inability to show the three-dimensional arrangement of atoms in space.
- Apply Lewis structures to predict molecular properties, such as polarity and reactivity.
Step-by-Step Guide to Drawing Lewis Structures
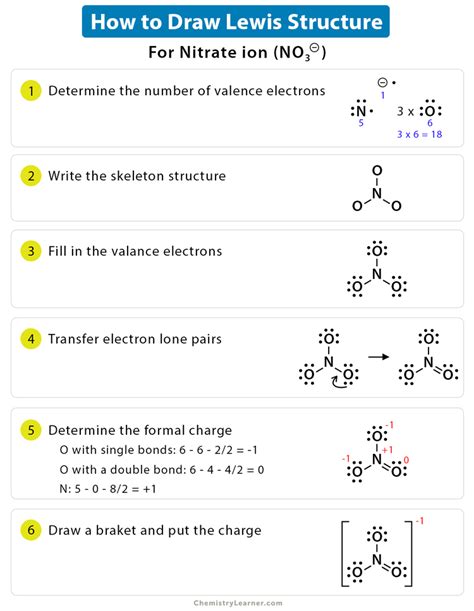
To draw a Lewis structure, follow these steps:
- Determine the total number of valence electrons in the molecule by summing the valence electrons of each atom. For main group elements, the number of valence electrons is equal to the group number in the periodic table.
- Identify the central atom, which is typically the least electronegative atom in the molecule. If the molecule contains hydrogen, it is usually not the central atom because hydrogen can only form one bond.
- Draw single bonds to the central atom from each of the surrounding atoms. This uses up two valence electrons for each bond.
- Distribute the remaining valence electrons as lone pairs around the atoms, making sure each atom has an octet (eight electrons) except for hydrogen, which has two electrons.
- Check for double or triple bonds if there are not enough electrons to give each atom an octet. Double and triple bonds use four and six electrons, respectively.
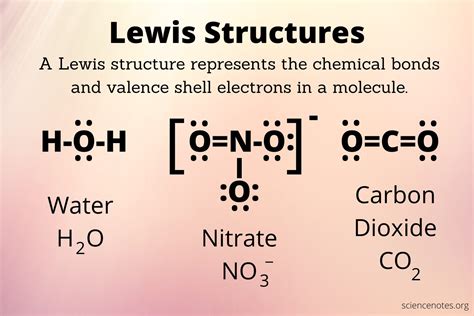
Example: Drawing the Lewis Structure of CO2
Carbon dioxide (CO2) is a simple molecule consisting of one carbon atom and two oxygen atoms. To draw its Lewis structure:
- The total number of valence electrons is 4 (from carbon) + 2*6 (from two oxygens) = 16.
- Carbon is the central atom because it is less electronegative than oxygen.
- Draw single bonds from carbon to each oxygen, using 4 electrons.
- Distribute the remaining 12 electrons as lone pairs on the oxygens and as double bonds between carbon and each oxygen to satisfy the octet rule for all atoms.
| Molecule | Central Atom | Total Valence Electrons |
|---|---|---|
| CO2 | Carbon | 16 |
| H2O | Oxygen | 8 |
| CH4 | Carbon | 8 |
Importance of Lewis Structures in Chemistry
Lewis structures are essential in chemistry because they help predict the shape of molecules, their polarity, and their reactivity. They are used in various fields, from organic chemistry to biochemistry, to understand how molecules interact with each other. By looking at a Lewis structure, chemists can determine if a molecule is polar or nonpolar, which affects its solubility and reactivity. Lewis structures also help in identifying the types of bonds (single, double, triple) and the presence of lone pairs, which are critical in understanding the chemical properties of a molecule.
Lewis Structures and Molecular Shape
The shape of a molecule can be predicted from its Lewis structure using VSEPR (Valence Shell Electron Pair Repulsion) theory. According to VSEPR, electron pairs (both bonding and lone pairs) around a central atom arrange themselves to minimize repulsion. For example, in a molecule with four bonding pairs and no lone pairs around the central atom, the shape is tetrahedral. Understanding the molecular shape is crucial for predicting physical and chemical properties, such as boiling point and reactivity.
Limitations of Lewis Structures
While Lewis structures are incredibly useful, they have limitations. They do not show the three-dimensional arrangement of electrons in space and do not account for delocalization of electrons, which can lead to a more stable structure than what the Lewis structure suggests. Additionally, Lewis structures do not predict the energy of the molecule or the strength of the bonds. For more complex molecules, especially those involving transition metals or where delocalization is significant, other methods like molecular orbital theory may provide a more accurate representation of the molecular structure.
What is the purpose of drawing Lewis structures?
+The purpose of drawing Lewis structures is to visualize the arrangement of electrons in a molecule, which helps in understanding its chemical properties and behavior.
How do you determine the central atom in a Lewis structure?
+The central atom is usually the least electronegative atom in the molecule, except for hydrogen, which typically forms only one bond and is not central.
What does the octet rule state in the context of Lewis structures?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell, which typically means having eight electrons in the valence shell, except for hydrogen, which seeks two electrons.
In conclusion, drawing Lewis structures is a fundamental skill in chemistry that, with practice and understanding, can provide valuable insights into the molecular structure and properties of substances. By following the steps outlined and practicing with different molecules, one can become proficient in drawing Lewis structures and apply this knowledge to predict and understand various chemical phenomena.