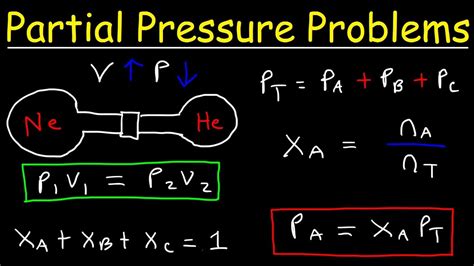
Calculating partial pressure is a fundamental concept in chemistry and physics, particularly in the study of gases. The partial pressure of a gas in a mixture is the pressure that the gas would exert if it were the only gas present in the container. This concept is crucial in understanding various phenomena, such as gas exchange in the lungs, the behavior of gases in industrial processes, and the environmental impact of gas emissions. In this article, we will delve into the world of partial pressures, exploring how to calculate them easily and efficiently.
Key Points
- The partial pressure of a gas can be calculated using Dalton's Law of Partial Pressures.
- The formula for calculating partial pressure is Pi = Ptotal * Xi, where Pi is the partial pressure of the gas, Ptotal is the total pressure of the mixture, and Xi is the mole fraction of the gas.
- The mole fraction of a gas can be determined by dividing the number of moles of the gas by the total number of moles of all gases in the mixture.
- Understanding partial pressures is essential in various fields, including respiratory medicine, environmental science, and chemical engineering.
- Calculating partial pressures can help predict the behavior of gases in different conditions and applications.
Understanding Dalton’s Law of Partial Pressures
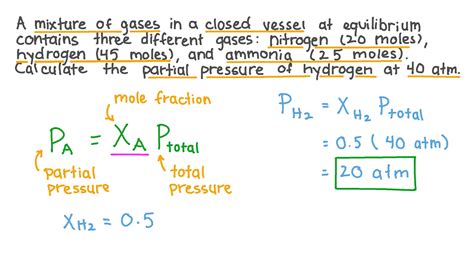
Dalton’s Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gas. This law is named after John Dalton, who first proposed it in the early 19th century. The law can be mathematically expressed as Ptotal = P1 + P2 +… + Pn, where Ptotal is the total pressure of the mixture, and P1, P2,…, Pn are the partial pressures of each gas.
Calculating Partial Pressure
The partial pressure of a gas can be calculated using the formula Pi = Ptotal * Xi, where Pi is the partial pressure of the gas, Ptotal is the total pressure of the mixture, and Xi is the mole fraction of the gas. The mole fraction of a gas is calculated by dividing the number of moles of the gas by the total number of moles of all gases in the mixture.
| Gas | Number of Moles | Mole Fraction | Partial Pressure (at 1 atm total pressure) |
|---|---|---|---|
| Oxygen (O2) | 0.21 | 0.21 | 0.21 atm |
| Nitrogen (N2) | 0.79 | 0.79 | 0.79 atm |
| Carbon Dioxide (CO2) | 0.01 | 0.01 | 0.01 atm |
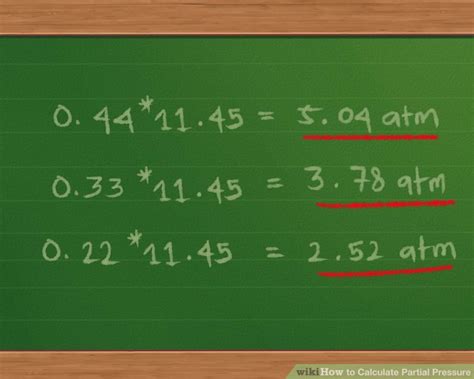
Applications of Partial Pressure Calculations
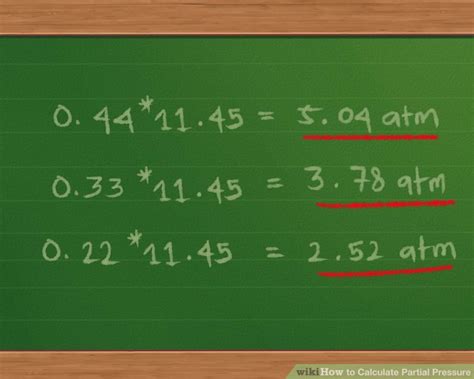
Calculating partial pressures has numerous applications in various fields, including medicine, environmental science, and chemical engineering. In respiratory medicine, understanding partial pressures is crucial in managing oxygen therapy and monitoring gas exchange in the lungs. In environmental science, partial pressure calculations help predict the behavior of greenhouse gases and their impact on climate change. In chemical engineering, partial pressures are used to design and optimize industrial processes, such as gas separation and purification.
Respiratory Medicine
In respiratory medicine, calculating partial pressures is essential in managing oxygen therapy and monitoring gas exchange in the lungs. The partial pressure of oxygen (pO2) and carbon dioxide (pCO2) in arterial blood is a critical parameter in assessing respiratory function and guiding treatment decisions.
Environmental Science
In environmental science, partial pressure calculations help predict the behavior of greenhouse gases and their impact on climate change. The partial pressure of carbon dioxide (CO2) in the atmosphere is a key indicator of global warming and climate change.
What is the formula for calculating partial pressure?
+The formula for calculating partial pressure is Pi = Ptotal * Xi, where Pi is the partial pressure of the gas, Ptotal is the total pressure of the mixture, and Xi is the mole fraction of the gas.
What is the mole fraction of a gas?
+The mole fraction of a gas is calculated by dividing the number of moles of the gas by the total number of moles of all gases in the mixture.
Why is calculating partial pressure important?
+Calculating partial pressure is important because it helps predict the behavior of gases in different conditions and applications, such as gas exchange in the lungs, the behavior of gases in industrial processes, and the environmental impact of gas emissions.
Meta Description: Calculate partial pressure easily using Dalton’s Law of Partial Pressures. Learn how to determine the mole fraction of a gas and apply it to various fields, including medicine, environmental science, and chemical engineering.