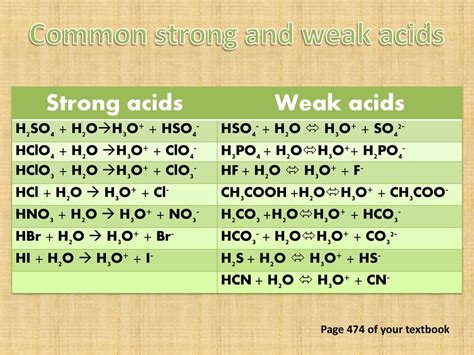
The classification of HNO3, or nitric acid, as a strong or weak acid is a fundamental concept in chemistry. To understand this, we must first delve into the definitions and characteristics of strong and weak acids. A strong acid is one that completely dissociates in water, producing a high concentration of hydrogen ions (H+). On the other hand, a weak acid only partially dissociates in water, resulting in a lower concentration of hydrogen ions.
Chemical Properties of HNO3
Nitric acid (HNO3) is a highly corrosive and toxic strong acid. Its chemical properties are characterized by its ability to fully dissociate in aqueous solutions. The dissociation reaction of HNO3 in water can be represented by the equation: HNO3 + H2O → H3O+ + NO3-. This complete dissociation indicates that HNO3 is a strong acid, as it fully ionizes in water to produce a high concentration of hydrogen ions (H3O+), which is a defining characteristic of strong acids.
Dissociation and Ionization
The strength of an acid is determined by its degree of dissociation in water. Strong acids, like HNO3, have a high degree of dissociation, meaning they almost completely ionize in aqueous solutions. This is in contrast to weak acids, which only partially dissociate. The complete dissociation of HNO3 in water is due to its molecular structure and the stability of its conjugate base, NO3- (nitrate ion). The nitrate ion is relatively stable, which facilitates the complete transfer of a proton (H+) from HNO3 to water, leading to the formation of hydronium ions (H3O+).
| Acid | Degree of Dissociation | Classification |
|---|---|---|
| HNO3 (Nitric Acid) | Complete | Strong Acid |
| HF (Hydrofluoric Acid) | Partial | Weak Acid |
| HCl (Hydrochloric Acid) | Complete | Strong Acid |

Implications of Being a Strong Acid
The classification of HNO3 as a strong acid has significant implications for its use and handling. Strong acids are highly reactive and can cause severe burns upon contact with skin. They also have the potential to react violently with bases and certain metals, releasing a large amount of heat and potentially toxic gases. In industrial and laboratory settings, the handling of strong acids like HNO3 requires careful consideration of safety protocols, including the use of protective equipment and controlled environments.
Applications of Nitric Acid
Nitric acid’s properties as a strong acid make it an essential reagent in various chemical processes. It is used in the production of ammonium nitrate, a common fertilizer, through its reaction with ammonia. Additionally, HNO3 is utilized in the manufacturing of explosives, dyes, and in the process of etching and cleaning metal surfaces. Its strong oxidizing properties also make it useful in the preparation of other acids and in organic synthesis reactions.
Key Points
- HNO3 (nitric acid) is classified as a strong acid due to its complete dissociation in water.
- The complete dissociation of HNO3 results in a high concentration of hydrogen ions, contributing to its strong acidic properties.
- Strong acids like HNO3 are highly reactive and require careful handling due to their potential to cause burns and violent reactions.
- HNO3 has various industrial applications, including the production of fertilizers, explosives, and in metal processing.
- Understanding the properties of strong acids like HNO3 is crucial for their safe and effective use in chemical processes.
In conclusion, the classification of HNO3 as a strong acid is based on its complete dissociation in water and its high reactivity. Its properties and applications underscore the importance of understanding the chemical behavior of acids in various industrial and laboratory contexts. By recognizing the characteristics of strong acids like HNO3, individuals can better appreciate their utility and the precautions necessary for their safe handling and use.
What is the primary characteristic that defines HNO3 as a strong acid?
+The primary characteristic that defines HNO3 as a strong acid is its complete dissociation in water, resulting in a high concentration of hydrogen ions.
What are some common applications of nitric acid due to its strong acid properties?
+Nitric acid is used in the production of fertilizers, explosives, and in metal processing, among other applications, due to its strong acidic and oxidizing properties.
Why is it important to handle strong acids like HNO3 with caution?
+Strong acids like HNO3 are highly reactive and can cause severe burns upon contact with skin, and they have the potential to react violently with bases and certain metals, making cautious handling essential.