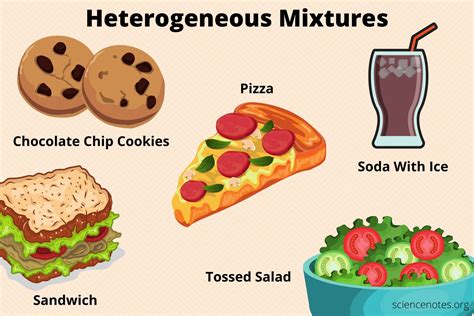
A heterogeneous mixture is a type of mixture that consists of two or more substances that are not uniformly distributed throughout the mixture. In other words, the components of a heterogeneous mixture are not mixed together at the molecular or atomic level, and they can be visually distinguished from one another. This is in contrast to homogeneous mixtures, where the components are uniformly distributed and cannot be visually distinguished.
The concept of heterogeneous mixtures is crucial in various fields, including chemistry, physics, and engineering. In chemistry, heterogeneous mixtures are often used to describe suspensions, colloids, and other types of mixtures where the components are not uniformly distributed. For instance, a mixture of sand and water is a heterogeneous mixture because the sand particles are not uniformly distributed throughout the water. Similarly, a mixture of oil and water is also a heterogeneous mixture because the two liquids do not mix together and can be separated by decantation or other methods.
Key Points
- A heterogeneous mixture consists of two or more substances that are not uniformly distributed throughout the mixture.
- The components of a heterogeneous mixture can be visually distinguished from one another.
- Heterogeneous mixtures are commonly found in nature and are used in various industrial applications.
- Examples of heterogeneous mixtures include suspensions, colloids, and mixtures of immiscible liquids.
- The properties of a heterogeneous mixture depend on the properties of its individual components and their relative proportions.
Characteristics of Heterogeneous Mixtures
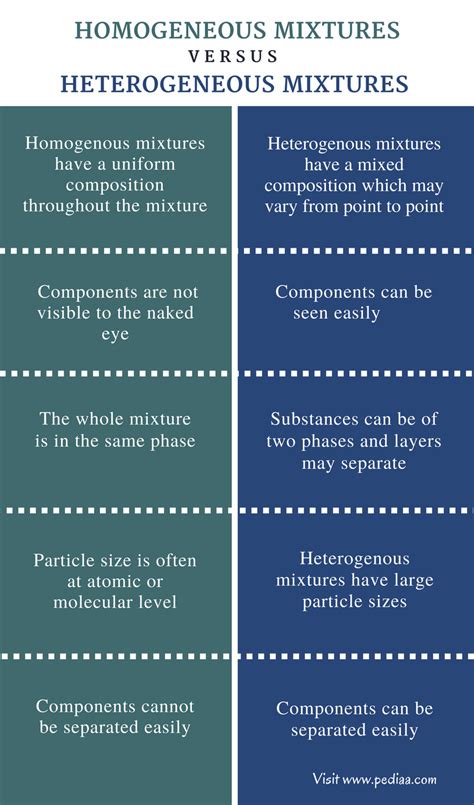
Heterogeneous mixtures have several distinct characteristics that set them apart from homogeneous mixtures. One of the most notable characteristics of heterogeneous mixtures is that they can be separated into their individual components by physical means, such as filtration, decantation, or centrifugation. For example, a mixture of sand and water can be separated by filtration, where the sand particles are retained by the filter paper and the water passes through.
Another characteristic of heterogeneous mixtures is that they can exhibit a range of properties, depending on the properties of their individual components and their relative proportions. For instance, a mixture of oil and water can exhibit different densities, viscosities, and refractive indices, depending on the proportion of each component. This is in contrast to homogeneous mixtures, where the properties of the mixture are uniform throughout.
Types of Heterogeneous Mixtures
There are several types of heterogeneous mixtures, including suspensions, colloids, and mixtures of immiscible liquids. Suspensions are mixtures of solid particles in a liquid or gas, where the particles are not uniformly distributed and can be separated by filtration or centrifugation. Examples of suspensions include mud, blood, and paint.
Colloids are mixtures of small particles in a liquid or gas, where the particles are not uniformly distributed but are too small to be seen with the naked eye. Examples of colloids include milk, fog, and smoke. Mixtures of immiscible liquids, such as oil and water, are also heterogeneous mixtures because the two liquids do not mix together and can be separated by decantation or other methods.
| Type of Mixture | Characteristics | Examples |
|---|---|---|
| Suspensions | Solid particles in a liquid or gas | Mud, blood, paint |
| Colloids | Small particles in a liquid or gas | Milk, fog, smoke |
| Mixtures of immiscible liquids | Two or more liquids that do not mix together | Oil and water, gasoline and water |
Applications of Heterogeneous Mixtures
Heterogeneous mixtures have a wide range of applications in various fields, including chemistry, physics, engineering, and biology. In chemistry, heterogeneous mixtures are used to study chemical reactions, phase transitions, and thermodynamic properties. For example, the study of heterogeneous mixtures is crucial in understanding the behavior of catalysts, which are used to speed up chemical reactions in industrial processes.
In physics, heterogeneous mixtures are used to study the properties of materials, such as their mechanical strength, thermal conductivity, and electrical conductivity. For instance, the study of colloids is essential in understanding the behavior of nanomaterials, which have unique properties due to their small size.
In engineering, heterogeneous mixtures are used to develop new materials and technologies, such as composites, coatings, and adhesives. For example, the development of composite materials, such as fiberglass and carbon fiber, relies on the understanding of heterogeneous mixtures and their properties.
Future Perspectives
The study of heterogeneous mixtures is an active area of research, with new discoveries and applications emerging every year. One of the most exciting areas of research is the development of new materials with unique properties, such as nanomaterials and metamaterials. These materials have the potential to revolutionize various industries, including energy, aerospace, and biotechnology.
Another area of research is the study of heterogeneous mixtures in biological systems, such as cells and tissues. Understanding the behavior of heterogeneous mixtures in these systems can provide insights into the underlying mechanisms of biological processes and diseases.
What is the difference between a homogeneous and heterogeneous mixture?
+A homogeneous mixture is a mixture where the components are uniformly distributed throughout the mixture, whereas a heterogeneous mixture is a mixture where the components are not uniformly distributed and can be visually distinguished from one another.
What are some examples of heterogeneous mixtures?
+Examples of heterogeneous mixtures include suspensions, colloids, and mixtures of immiscible liquids, such as oil and water, mud, blood, and paint.
What are some applications of heterogeneous mixtures?
+Heterogeneous mixtures have a wide range of applications in various fields, including chemistry, physics, engineering, and biology, such as the development of new materials, catalysts, and technologies.