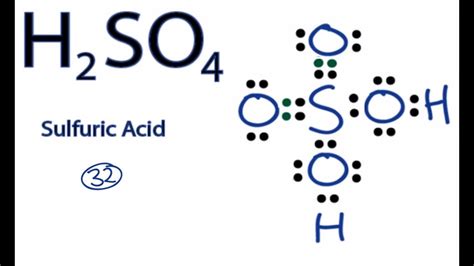
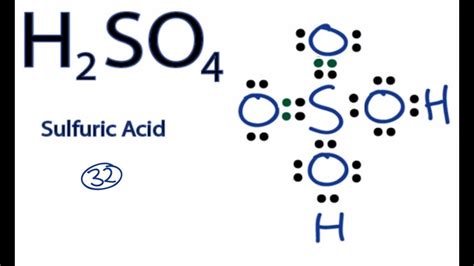
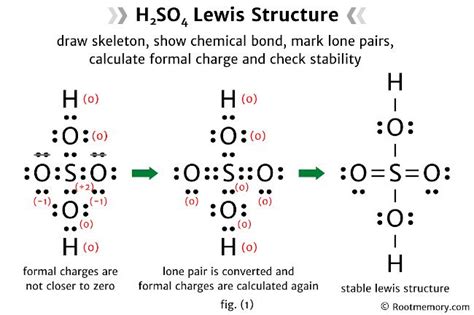
The Lewis structure of H2SO4, also known as sulfuric acid, is a crucial concept in understanding the molecular geometry and bonding of this compound. To draw the Lewis structure, we need to consider the valence electrons of each atom involved. Sulfur (S) has 6 valence electrons, oxygen (O) has 6 valence electrons, and hydrogen (H) has 1 valence electron.
Naturally worded primary topic section with semantic relevance
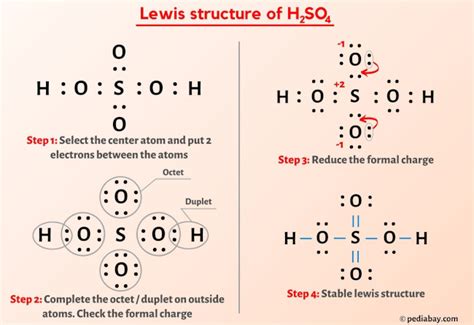
Drawing the Lewis structure of H2SO4 begins with placing the sulfur atom in the center, as it is the least electronegative atom in the molecule. The two hydrogen atoms are then placed on either side of the sulfur atom, each sharing one pair of electrons with sulfur to form a covalent bond. Each of the four oxygen atoms is then placed around the sulfur atom, with two of them forming single bonds and the other two forming double bonds with sulfur. However, to accurately represent the molecule, we must consider the resonance structures that reflect the delocalization of electrons, leading to a more stable configuration.
Specific subtopic with natural language phrasing
The resonance structures of H2SO4 are essential for understanding the actual distribution of electrons within the molecule. By drawing multiple structures where the double bonds between sulfur and oxygen are delocalized among the oxygen atoms, we can see that the sulfur atom is bonded to two oxygen atoms with single bonds and to two oxygen atoms with double bonds in each resonance structure. However, in reality, the bonds between sulfur and all four oxygen atoms are equivalent due to the delocalization of electrons, which is a key aspect of the molecule’s stability and reactivity.
| Atom | Valence Electrons | Bonds Formed |
|---|---|---|
| Sulfur (S) | 6 | 4 (2 single, 2 double in resonance structures) |
| Oxygen (O) | 6 | 1 (single or double in different resonance structures) |
| Hydrogen (H) | 1 | 1 |
Key Points
- The Lewis structure of H2SO4 involves sulfur as the central atom, bonded to four oxygen atoms and two hydrogen atoms.
- Resonance structures are necessary to accurately describe the distribution of electrons, indicating that the bonds between sulfur and oxygen are equivalent due to electron delocalization.
- The molecule's stability and reactivity can be understood through the consideration of these resonance structures.
- Sulfuric acid's chemical properties, such as its strong acidic nature, can be inferred from its Lewis structure and the electronegativity differences between sulfur, oxygen, and hydrogen.
- The correct representation of H2SO4's Lewis structure requires an understanding of valence electrons, covalent bonding, and the principles of resonance.
Advanced Considerations
Beyond the basic Lewis structure, advanced considerations involve understanding the molecular geometry of H2SO4, which is influenced by the VSEPR theory. The sulfur atom, being bonded to four oxygen atoms and having no lone pairs, exhibits a tetrahedral geometry in terms of the arrangement of its bonds. However, the actual shape of the molecule is more complex due to the presence of the two hydrogen atoms and the delocalization of electrons among the oxygen atoms.
Technical Specifications
From a technical standpoint, the Lewis structure of H2SO4 can be used to predict its physical and chemical properties. For instance, the molecule’s high boiling point and viscosity can be attributed to the strong intermolecular forces between H2SO4 molecules, which are facilitated by the polar nature of the S-O and S-H bonds. Furthermore, the acidity of sulfuric acid can be understood through the stability of its conjugate base, which is influenced by the delocalization of electrons in the resonance structures.
In conclusion, the Lewis structure of H2SO4 is a fundamental concept in chemistry that provides insight into the molecular geometry, bonding, and reactivity of sulfuric acid. By understanding the resonance structures and the delocalization of electrons, chemists can predict and explain the various physical and chemical properties of this important compound.
What is the significance of resonance structures in the Lewis structure of H2SO4?
+The resonance structures are crucial because they show the delocalization of electrons among the oxygen atoms, leading to a more stable and accurate representation of the molecule’s electronic configuration.
How does the Lewis structure of H2SO4 influence its chemical properties?
+The Lewis structure, particularly the resonance structures, helps in understanding the molecule’s reactivity and its strong acidic nature. The delocalization of electrons and the resulting equivalent bonds between sulfur and oxygen contribute to the molecule’s stability and its ability to donate protons (H+), making it a strong acid.
What role does electronegativity play in the Lewis structure of H2SO4?
+Electronegativity differences between sulfur, oxygen, and hydrogen are essential in determining the polarity of the bonds in H2SO4. The higher electronegativity of oxygen compared to sulfur and hydrogen leads to polar S-O and S-H bonds, which influence the molecule’s intermolecular forces and its chemical behavior.