The H2O Lewis structure is a fundamental concept in chemistry, representing the molecular structure of water. To understand this structure, it's essential to grasp the basics of Lewis structures and how they are constructed. A Lewis structure is a diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons that may exist. It's a crucial tool for predicting the shape and reactivity of molecules.
Understanding the Basics of Lewis Structures
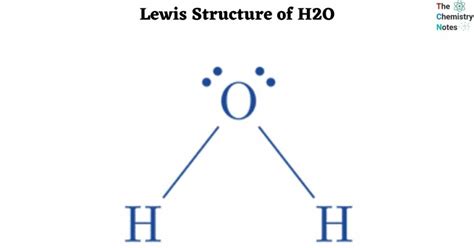
Before diving into the H2O Lewis structure, let’s review the steps to create a Lewis structure. First, determine the total number of valence electrons in the molecule. For H2O, this involves adding the valence electrons of two hydrogen atoms (1 each) and one oxygen atom (6). Next, draw the atoms and connect them with single bonds, which represents the sharing of two electrons between atoms. Finally, distribute the remaining valence electrons as lone pairs around the atoms, ensuring that each atom has a full outer shell, typically 8 electrons for non-hydrogen atoms, following the octet rule.
Step 1: Determine the Total Number of Valence Electrons
The total number of valence electrons for H2O is calculated by adding the valence electrons of hydrogen (1*2) and oxygen (6), resulting in 8 valence electrons.
| Atom | Valence Electrons |
|---|---|
| Hydrogen (H) | 1 |
| Oxygen (O) | 6 |
| Total | 8 |
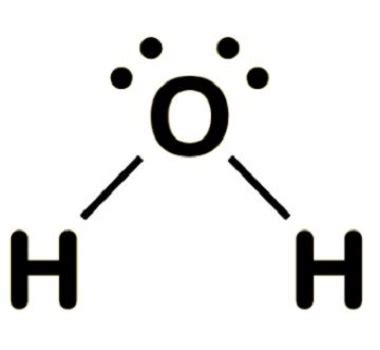
Constructing the H2O Lewis Structure
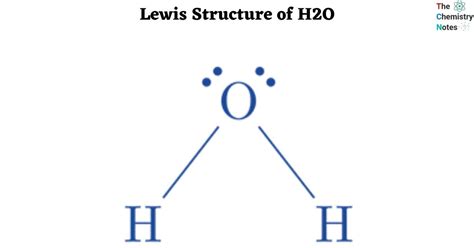
To construct the H2O Lewis structure, start by placing the oxygen atom in the center, as it is the least electronegative atom and can form more than one bond. Then, arrange the two hydrogen atoms around the oxygen, connecting each hydrogen to the oxygen with a single bond. This uses 4 of the 8 valence electrons. The remaining 4 electrons are distributed around the oxygen atom as two lone pairs, ensuring the oxygen has a full outer shell and adhering to the octet rule.
Step 2: Draw the Atoms and Connect with Single Bonds
With oxygen in the center, the two hydrogen atoms are connected to it with single bonds. This step utilizes 4 valence electrons, leaving 4 electrons to be distributed as lone pairs around the oxygen atom.
Key Points
- The oxygen atom is placed in the center due to its ability to form multiple bonds.
- Two hydrogen atoms are connected to the oxygen with single bonds, using 4 valence electrons.
- The remaining 4 electrons are distributed as two lone pairs around the oxygen atom.
- The resulting structure adheres to the octet rule for all atoms involved.
- The H2O molecule exhibits a bent or V-shape due to the presence of lone pairs on the oxygen atom.
Understanding the Shape of H2O
The shape of the H2O molecule, based on its Lewis structure, is bent or V-shaped. This shape is a result of the lone pairs on the oxygen atom, which occupy space and cause the molecule to bend. The angle between the two hydrogen atoms is approximately 104.5 degrees, which is less than the 109.5 degrees expected for a tetrahedral arrangement due to the repulsion between the lone pairs and the bonding pairs of electrons.
Implications of the Bent Shape
The bent shape of H2O has significant implications for its physical and chemical properties. It makes water a polar molecule, with the oxygen end having a partial negative charge and the hydrogen ends having partial positive charges. This polarity is crucial for the solvent properties of water and its ability to form hydrogen bonds with other water molecules, which influences its boiling point, surface tension, and other characteristics.
What is the total number of valence electrons in the H2O molecule?
+The total number of valence electrons in H2O is 8, calculated by adding 2*1 (for the two hydrogen atoms) and 6 (for the oxygen atom).
Why does the H2O molecule have a bent shape?
+The H2O molecule has a bent shape due to the presence of two lone pairs on the oxygen atom, which repel the bonding pairs of electrons and cause the molecule to bend, resulting in an approximate angle of 104.5 degrees between the hydrogen atoms.
In conclusion, understanding the H2O Lewis structure is essential for grasping the molecular properties of water. The construction of the Lewis structure, following the octet rule and considering the electronegativity of atoms, leads to a bent molecular shape. This shape, in turn, influences the polarity and hydrogen-bonding capabilities of water, making it a unique and vital solvent in chemical and biological processes.