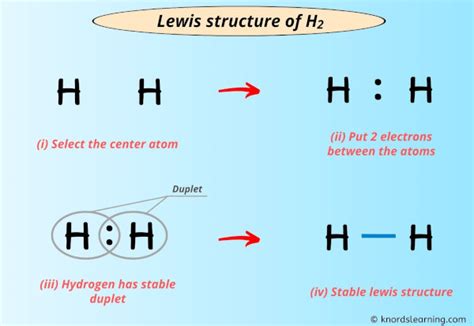
The H2 Lewis structure is a fundamental concept in chemistry, representing the molecular structure of a hydrogen molecule. It is a crucial aspect of understanding chemical bonding and molecular geometry. In this article, we will explore the H2 Lewis structure in depth, discussing its key aspects, importance, and applications.
Introduction to H2 Lewis Structure

The H2 molecule consists of two hydrogen atoms, each with one electron in its 1s orbital. When these two atoms come together, they form a covalent bond, resulting in a stable molecule. The Lewis structure of H2 is a simple representation of this molecule, showing the arrangement of electrons and bonds between the atoms. It is essential to understand the H2 Lewis structure to grasp the principles of chemical bonding and molecular interactions.
Key Aspects of H2 Lewis Structure
The H2 Lewis structure has several key aspects that are important to understand: 1. Electron Configuration: Each hydrogen atom has one electron in its 1s orbital, which is the lowest energy level. When the two atoms come together, they share their electrons to form a covalent bond. 2. Bond Order: The bond order of H2 is 1, indicating a single covalent bond between the two atoms. 3. Bond Length: The bond length of H2 is approximately 74 pm, which is the average distance between the two nuclei. 4. Molecular Geometry: The molecular geometry of H2 is linear, with the two atoms arranged in a straight line. 5. Electron Density: The electron density of H2 is evenly distributed between the two atoms, resulting in a symmetrical molecule.
Key Points
- The H2 Lewis structure represents the molecular structure of a hydrogen molecule.
- The molecule consists of two hydrogen atoms, each with one electron in its 1s orbital.
- The bond order of H2 is 1, indicating a single covalent bond between the two atoms.
- The bond length of H2 is approximately 74 pm.
- The molecular geometry of H2 is linear, with the two atoms arranged in a straight line.
Importance of H2 Lewis Structure
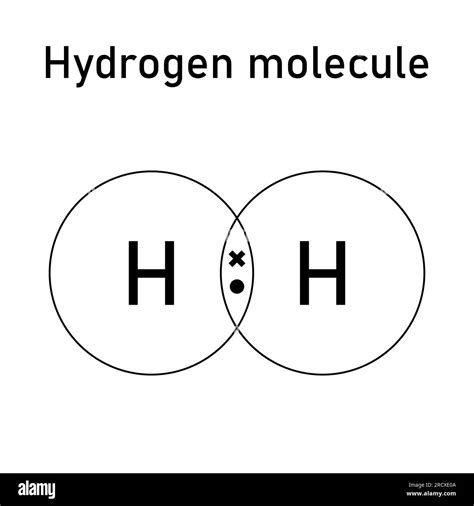
The H2 Lewis structure is crucial in understanding various chemical concepts, including: 1. Chemical Bonding: The H2 Lewis structure helps explain the formation of covalent bonds between atoms. 2. Molecular Geometry: The linear geometry of H2 is a result of the covalent bond between the two atoms. 3. Chemical Reactions: The H2 Lewis structure is essential in understanding various chemical reactions, including combustion and hydrogenation reactions. 4. Quantum Mechanics: The H2 molecule is a simple system that can be used to demonstrate the principles of quantum mechanics, including wave functions and probability densities. 5. Materials Science: The H2 Lewis structure is relevant in understanding the properties of materials, including their strength, conductivity, and reactivity.
| Property | Value |
|---|---|
| Bond Order | 1 |
| Bond Length | 74 pm |
| Molecular Geometry | Linear |
| Electron Density | Evenly distributed |

Applications of H2 Lewis Structure
The H2 Lewis structure has numerous applications in various fields, including: 1. Chemical Synthesis: The H2 Lewis structure is used to design and optimize chemical synthesis routes, including the production of hydrogen gas. 2. Materials Science: The H2 Lewis structure is relevant in understanding the properties of materials, including their strength, conductivity, and reactivity. 3. Energy Storage: The H2 Lewis structure is essential in understanding the properties of hydrogen storage materials, including their capacity, stability, and reactivity. 4. Quantum Computing: The H2 molecule is a simple system that can be used to demonstrate the principles of quantum computing, including quantum entanglement and superposition. 5. Chemical Education: The H2 Lewis structure is a fundamental concept in chemistry education, helping students understand the principles of chemical bonding and molecular geometry.
What is the bond order of H2?
+The bond order of H2 is 1, indicating a single covalent bond between the two atoms.
What is the molecular geometry of H2?
+The molecular geometry of H2 is linear, with the two atoms arranged in a straight line.
What are the applications of H2 Lewis structure?
+The H2 Lewis structure has numerous applications in various fields, including chemical synthesis, materials science, energy storage, quantum computing, and chemical education.
Meta Description: Learn about the H2 Lewis structure, its key aspects, importance, and applications in chemistry and materials science. Discover how the H2 Lewis structure is used to explain chemical bonding, molecular geometry, and chemical reactivity.