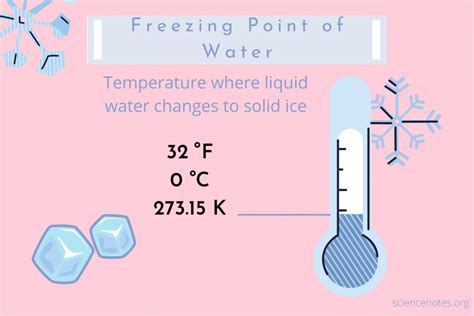
Water, a vital component of our planet, undergoes a fascinating transformation when its temperature drops to a specific point. At zero degrees Celsius, or 32 degrees Fahrenheit, water undergoes a phase transition, changing from a liquid to a solid state. This process, known as freezing, is a fundamental concept in physics and chemistry, with significant implications for various fields, including environmental science, biology, and engineering. The freezing point of water is a universally accepted standard, serving as a reference point for temperature measurements in the Celsius scale.
The Science Behind Freezing

The freezing of water is a complex process, involving the rearrangement of hydrogen bonds between water molecules. As the temperature of water decreases, the molecules slow down and come closer together, forming a crystalline structure. This structure, also known as ice, is characterized by a repeating pattern of hydrogen bonds, which provide the necessary stability for the solid state to exist. The freezing point of water is influenced by factors such as pressure, with higher pressures resulting in a lower freezing point. However, at standard atmospheric pressure, zero degrees Celsius remains the benchmark for the freezing of water.
Phase Transitions and Thermodynamics
Phase transitions, such as the freezing of water, are governed by the principles of thermodynamics. The process involves a change in the state of matter, from liquid to solid, which is accompanied by a release of energy in the form of latent heat. This energy is required to break the hydrogen bonds between water molecules, allowing them to rearrange and form the crystalline structure of ice. The thermodynamic properties of water, including its specific heat capacity and enthalpy of fusion, play a crucial role in determining the freezing point and the overall phase transition process.
| Property | Value |
|---|---|
| Freezing Point (°C) | 0 |
| Boiling Point (°C) | 100 |
| Specific Heat Capacity (J/g·K) | 4.184 |
| Enthalpy of Fusion (kJ/mol) | 6.01 |

Key Points
- The freezing point of water is zero degrees Celsius, or 32 degrees Fahrenheit, at standard atmospheric pressure.
- The freezing process involves a phase transition, characterized by the rearrangement of hydrogen bonds between water molecules.
- Thermodynamic properties, such as specific heat capacity and enthalpy of fusion, play a crucial role in determining the freezing point and phase transition process.
- Understanding the science behind freezing is essential for various industrial and scientific applications, including ice production, food preservation, and climate change research.
- The freezing point of water serves as a reference point for temperature measurements in the Celsius scale, highlighting its significance in the field of physics and chemistry.
Implications and Applications
The freezing of water has significant implications for various fields, including environmental science, biology, and engineering. In environmental science, the freezing of water plays a crucial role in shaping our planet’s climate and ecosystems. The formation of ice and snow influences global temperature patterns, weather events, and the distribution of water resources. In biology, the freezing of water is essential for the preservation of food and the study of cryopreservation, which involves the use of low temperatures to preserve biological samples.
Ice Formation and Climate Change
The formation of ice is a critical aspect of climate change research, as it influences global temperature patterns and weather events. The freezing of water in polar regions, such as the Arctic and Antarctica, plays a significant role in regulating the Earth’s climate, with changes in ice coverage and thickness impacting global temperature patterns and sea levels. Understanding the science behind ice formation and the freezing of water is essential for predicting and mitigating the effects of climate change.
In conclusion, the freezing of water at zero degrees Celsius is a complex process, governed by the principles of thermodynamics and influenced by factors such as pressure and temperature. The science behind freezing has significant implications for various fields, including environmental science, biology, and engineering, highlighting the importance of understanding this fundamental concept in physics and chemistry.
What is the freezing point of water at standard atmospheric pressure?
+The freezing point of water at standard atmospheric pressure is zero degrees Celsius, or 32 degrees Fahrenheit.
What is the role of hydrogen bonds in the freezing of water?
+Hydrogen bonds between water molecules play a crucial role in the freezing of water, as they provide the necessary stability for the solid state to exist. The rearrangement of hydrogen bonds is essential for the formation of ice crystals.
How does the freezing of water influence climate change?
+The freezing of water in polar regions, such as the Arctic and Antarctica, plays a significant role in regulating the Earth’s climate, with changes in ice coverage and thickness impacting global temperature patterns and sea levels.