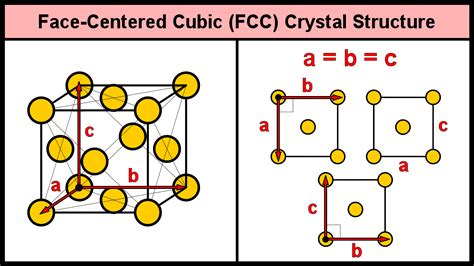
The face-centered cubic (FCC) structure is one of the most common and significant crystal structures found in metals and other materials. It is a fundamental concept in materials science and solid-state physics, and understanding its properties and characteristics is essential for a wide range of applications, from engineering to nanotechnology. In this article, we will delve into the details of the FCC structure, exploring its definition, properties, and significance, as well as its relevance to various fields of study.
The face-centered cubic structure is a type of crystal lattice where each unit cell consists of a cube with atoms located at the corners and the centers of each face. This arrangement is also known as a cubic close-packed (CCP) structure, and it is characterized by a high packing density, with each atom surrounded by 12 nearest neighbors. The FCC structure is commonly found in metals such as copper, silver, and gold, as well as in other materials like silicon and diamond.
Crystal Structure and Lattice Parameters

The FCC structure can be described by a set of lattice parameters, which define the size and shape of the unit cell. The lattice parameters of an FCC crystal are typically denoted by the symbol “a,” which represents the length of the edge of the cube. The lattice parameters of an FCC crystal can be calculated using the following formula: a = 2 * (r / √2), where r is the radius of the atom. The lattice parameters of an FCC crystal are important because they determine the physical properties of the material, such as its density, elastic modulus, and thermal expansion coefficient.
Key Points
- The face-centered cubic structure is a type of crystal lattice with atoms located at the corners and centers of each face.
- The FCC structure is characterized by a high packing density, with each atom surrounded by 12 nearest neighbors.
- The lattice parameters of an FCC crystal are typically denoted by the symbol "a," which represents the length of the edge of the cube.
- The FCC structure is commonly found in metals such as copper, silver, and gold, as well as in other materials like silicon and diamond.
- The physical properties of an FCC crystal, such as its density, elastic modulus, and thermal expansion coefficient, are determined by its lattice parameters.
Properties of Face-Centered Cubic Structures
The face-centered cubic structure has several important properties that make it useful for a wide range of applications. One of the most significant properties of an FCC crystal is its high packing density, which is the ratio of the volume of the atoms to the total volume of the crystal. The packing density of an FCC crystal is typically around 74%, which is higher than that of other crystal structures, such as the body-centered cubic (BCC) structure. The high packing density of an FCC crystal gives it a high density, which is important for applications such as aerospace and biomedical engineering.Another important property of an FCC crystal is its high ductility, which is the ability of the material to deform without breaking. The high ductility of an FCC crystal is due to the presence of multiple slip systems, which are planes of atoms that can slide past each other when the material is subjected to stress. The high ductility of an FCC crystal makes it useful for applications such as wire drawing and metal forming.
| Property | Value |
|---|---|
| Packing Density | 74% |
| Ductility | High |
| Density | Dependent on material |
| Elastic Modulus | Dependent on material |
| Thermal Expansion Coefficient | Dependent on material |

Applications of Face-Centered Cubic Structures

The face-centered cubic structure has a wide range of applications, from aerospace to biomedical engineering. One of the most significant applications of an FCC crystal is in the field of materials science, where it is used to develop new materials with specific properties. For example, the FCC structure is used in the development of high-strength, low-alloy (HSLA) steels, which are used in applications such as bridge construction and shipbuilding.
Another important application of an FCC crystal is in the field of nanotechnology, where it is used to develop new materials with unique properties. For example, the FCC structure is used in the development of nanoparticles, which are used in applications such as cancer treatment and drug delivery.
The face-centered cubic structure is also used in the field of electronics, where it is used to develop new materials with specific electrical properties. For example, the FCC structure is used in the development of semiconductors, which are used in applications such as computer chips and solar cells.
Comparison with Other Crystal Structures
The face-centered cubic structure is one of several crystal structures that are commonly found in metals and other materials. Other common crystal structures include the body-centered cubic (BCC) structure, the hexagonal close-packed (HCP) structure, and the diamond cubic structure. Each of these crystal structures has its own unique properties and characteristics, and is suited to specific applications.For example, the BCC structure is commonly found in metals such as iron and chromium, and is characterized by a lower packing density than the FCC structure. The HCP structure is commonly found in metals such as titanium and zinc, and is characterized by a higher packing density than the BCC structure. The diamond cubic structure is commonly found in materials such as silicon and diamond, and is characterized by a very high packing density and a unique set of electrical properties.
What is the face-centered cubic structure?
+The face-centered cubic structure is a type of crystal lattice where each unit cell consists of a cube with atoms located at the corners and the centers of each face.
What are the properties of the face-centered cubic structure?
+The face-centered cubic structure has several important properties, including a high packing density, high ductility, and high density.
What are the applications of the face-centered cubic structure?
+The face-centered cubic structure has a wide range of applications, from aerospace to biomedical engineering, and is used in the development of new materials with specific properties.
In conclusion, the face-centered cubic structure is a fundamental concept in materials science and solid-state physics, with a wide range of applications in fields such as aerospace, biomedical engineering, and nanotechnology. Its high packing density, high ductility, and high density make it an ideal material for a wide range of uses, and its unique properties and characteristics make it an important area of study for researchers and engineers. By understanding the properties and applications of the face-centered cubic structure, we can develop new materials and technologies that have the potential to transform a wide range of industries and improve our daily lives.