When it comes to understanding the complex world of cycloaddition reactions, a fundamental grasp of the difference between endo and exo selectivity is crucial. In organic chemistry, the terms endo and exo refer to the stereochemical outcome of a reaction, particularly in the context of Diels-Alder reactions and other cycloaddition processes. The distinction between these two terms is not merely semantic; it has profound implications for the synthesis of complex molecules, including pharmaceuticals and natural products. In this article, we will delve into the nuances of endo vs exo selectivity, exploring the theoretical underpinnings, practical applications, and expert insights that underscore the importance of this concept in modern organic synthesis.
Understanding Endo and Exo Selectivity
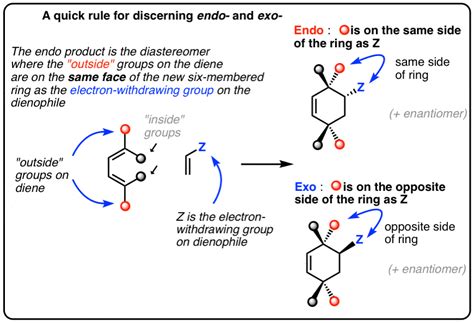
The terms endo and exo are derived from the Greek words for “inside” and “outside,” respectively. In the context of cycloaddition reactions, endo refers to the orientation where the substituents on the dienophile are positioned “inside” or cis to the newly formed ring, whereas exo refers to the orientation where these substituents are positioned “outside” or trans to the newly formed ring. This distinction is critical because the stereochemical outcome of a reaction can significantly influence the physical, chemical, and biological properties of the resulting compound. For instance, in the synthesis of complex natural products, achieving the correct stereochemistry is often the key to unlocking the desired biological activity.
Theoretical Foundations
Theoretical models, such as the Frontier Molecular Orbital (FMO) theory, provide a framework for understanding the factors that influence endo vs exo selectivity. According to FMO theory, the interaction between the highest occupied molecular orbital (HOMO) of the diene and the lowest unoccupied molecular orbital (LUMO) of the dienophile plays a pivotal role in determining the stereochemical outcome of the reaction. Factors such as the size and electronegativity of substituents on both the diene and dienophile can influence the energy of these orbitals, thereby affecting the endo/exo ratio. Additionally, solvent effects and reaction conditions, including temperature and pressure, can also impact the selectivity of the reaction.
| Reaction Conditions | Endo/Exo Ratio |
|---|---|
| High Temperature | Decreased Endo Selectivity |
| Polar Solvent | Increased Endo Selectivity |
| High Pressure | Increased Endo Selectivity |
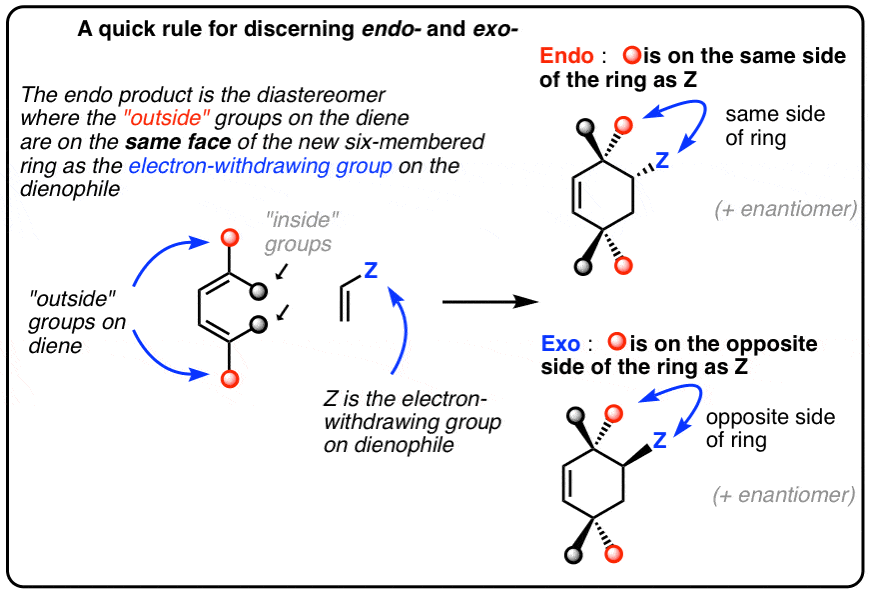
Practical Applications and Tips
In practical terms, controlling endo vs exo selectivity is a challenging but crucial aspect of organic synthesis. Here are five expert tips for achieving high selectivity in cycloaddition reactions:
- Substituent Effects: The strategic placement of substituents on the diene and dienophile can significantly influence the endo/exo ratio. Bulky substituents, for example, can sterically hinder the exo approach, favoring the endo product.
- Solvent Effects: The choice of solvent can have a profound impact on the reaction outcome. Polar solvents, in particular, can enhance endo selectivity by stabilizing the transition state leading to the endo product.
- Temperature Control: Reaction temperature is a critical parameter in controlling selectivity. Lower temperatures often favor the endo product by reducing the energy available for the reaction, thereby minimizing the formation of the less stable exo transition state.
- Pressure Influence: High pressure can significantly influence the endo/exo ratio by favoring the more compact, endo transition state. This can be a powerful tool in achieving high selectivity, especially in reactions where steric effects are significant.
- Catalyst Selection: The use of catalysts, such as Lewis acids, can also modulate the endo/exo selectivity. These catalysts can complex with the dienophile, altering its LUMO energy and thereby influencing the reaction outcome.
Key Points
- Understanding the theoretical underpinnings of endo vs exo selectivity is crucial for predicting reaction outcomes.
- Substituent effects, solvent effects, temperature control, pressure influence, and catalyst selection are critical factors in achieving high selectivity.
- A nuanced approach to reaction conditions and molecular design is essential for controlling the stereochemical outcome of cycloaddition reactions.
- Expertise in manipulating these factors can significantly enhance the efficiency and selectivity of organic syntheses.
- Continuous advancements in theoretical models and experimental techniques are expanding our ability to predict and control endo/exo selectivity, enabling the synthesis of increasingly complex molecules.
In conclusion, the distinction between endo and exo selectivity is a fundamental concept in organic chemistry, with profound implications for the synthesis of complex molecules. By understanding the theoretical foundations and practical applications of this concept, chemists can develop sophisticated strategies for controlling the stereochemical outcome of cycloaddition reactions, thereby advancing the field of organic synthesis.
What is the primary factor influencing endo vs exo selectivity in cycloaddition reactions?
+The primary factor is the interaction between the molecular orbitals of the diene and dienophile, as described by the Frontier Molecular Orbital theory. However, reaction conditions such as solvent, temperature, and pressure also play significant roles.
How can substituents on the diene and dienophile influence the endo/exo ratio?
+Substituents can influence the endo/exo ratio through steric and electronic effects. Bulky substituents can sterically hinder the exo approach, favoring the endo product, while electron-withdrawing or electron-donating groups can influence the energy of the molecular orbitals involved in the reaction.
What role does solvent play in controlling endo vs exo selectivity?
+Solvent can play a crucial role by stabilizing the transition state leading to the endo or exo product. Polar solvents, for example, can enhance endo selectivity by stabilizing the more polar endo transition state.